

Corporate Presentation October 2025

Disclaimer This presentation, including any printed or electronic copy of these slides, the talks given by the presenters, the information communicated during any delivery of the presentation and any question and answer session and any document distributed at or in connection with the presentation (collectively, the “Presentation”) has been prepared by Cabaletta Bio, Inc. (“we,” “us,” “our,” “Cabaletta” or the “Company”) and may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, and include, but are not limited to, express or implied statements regarding our current beliefs, expectations and assumptions regarding: our business, future plans and strategies for our technology; our ability to grow our autoimmune-focused pipeline; the ability to capitalize on and potential benefits resulting from our research and translational insights, including those related to any similarly-designed constructs or dosing regimens; the anticipated market opportunities for rese-cel in patients with autoimmune diseases; the Company's business plans and objectives; our expectations around the potential success and therapeutic and clinical benefits of rese-cel, as well as our ability to successfully complete research and further development and commercialization of our drug candidates in current or future indications, including the timing and results of our clinical trials and our ability to conduct and complete clinical trials; expectation that clinical results will support rese-cel's safety and activity profile; our plan to leverage increasing clinical data and a unique development program for rese-cel; the timing, clinical significance and impact of clinical data read-outs, including the progress, results and clinical data from each of the patients dosed with rese-cel in the Phase 1/2 RESET-Myositis, RESET-SLE, RESET-SSc, RESET-MG and RESET-PV trials and our other planned activities with respect to rese-cel; our belief that rese-cel has the potential to provide drug-free, durable transformative clinical responses, through an immune reset, including the potential for achieving drug-free remission in patients with refractory myositis; the Company's advancement of separate Phase 1/2 clinical trials of rese-cel and advancement of the RESET-PV and RESET-MS trials, with and without preconditioning, as applicable, including updates related to status, safety data, efficiency of clinical trial design and timing of data read-outs or otherwise; our ability to leverage our experience in autoimmune cell therapy; our ability to enroll the requisite number of patients, dose each dosing cohort in the intended manner and timing thereof, and advance the trial as planned in our Phase 1/2 clinical trials of rese-cel; the timing any planned regulatory filings for our development programs, including IND applications and interactions with regulatory authorities, including such authorities' review of safety information from our ongoing clinical trials and alignment with regulatory agencies on potential registrational pathway for rese-cel in various indications, and the timing of alignment of trial design related thereto; our ability to successfully complete our preclinical and clinical studies for our product candidates, including our ability to progress the trial; Cabaletta's advancement of the whole blood manufacturing program to remove the burden of apheresis; our ability to increase enrollment from our rapidly expanding clinical network in the RESET clinical trial program in the US and Europe; our ability to obtain and maintain regulatory approval of our product candidates, including our expectations regarding the intended incentives conferred by and ability to retain regulatory designations and the anticipated initiation of registrational cohorts and potential BLA submission; our expectation and timing for completion of dosing of most disease-specific cohorts; our belief regarding alignment with FDA on registrational trial design and timing thereof; our expectations regarding opportunities based on market research; our ability to accelerate our pipeline to approval and launch and to develop transformative therapies for patients, including in collaboration with academic and industry partners and the ability to optimize such collaborations on, including timing thereof, our development programs; our ability to contract with third-party suppliers and manufacturers; our ability to execute our manufacturing strategy to enable expansion of clinical supply and efficiently scale commercial supply for rese-cel; our potential commercial opportunities, including value and addressable market, for our product candidates. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Various risks, uncertainties and assumptions could cause actual results to differ materially from those anticipated or implied in our forward-looking statements. Such risks and uncertainties include, but are not limited to, risks related to the success, cost, and timing of our development activities and clinical trials, risks related to our ability to demonstrate sufficient evidence of safety, efficacy and tolerability in our clinical trials, the risk that the results observed with the similarly-designed construct, including, but not limited to, dosing regimen, are not indicative of the results we seek to achieve with rese-cel, the risk that signs of biologic activity or persistence may not inform long-term results, risks related to clinical trial site activation or enrollment rates that are lower than expected, risks that modifications to trial design or approach may not have the intended benefits and that the trial design may need to be further modified; our ability to protect and maintain our intellectual property position, risks related to our relationships with third parties, uncertainties related to regulatory agencies’ evaluation of regulatory filings and other information related to our product candidates, our ability to retain and recognize the intended incentives conferred by any regulatory designations, risks related to regulatory filings and potential clearance, the risk that any one or more of our product candidates will not be successfully developed and commercialized, the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies, risks related to volatile market and economic conditions and our ability to fund operations and continue as a going concern. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ materially from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our most recent annual report on Form 10-K and quarterly report on Form 10-Q, as well as discussions of potential risks, uncertainties, and other important factors in our other filings with the Securities and Exchange Commission. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. The Company is the owner of various trademarks, trade names and service marks. Certain other trademarks, trade names and service marks appearing in this Presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this Presentation are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.

Develop and launch the first curative targeted cellular therapies for patients with autoimmune diseases
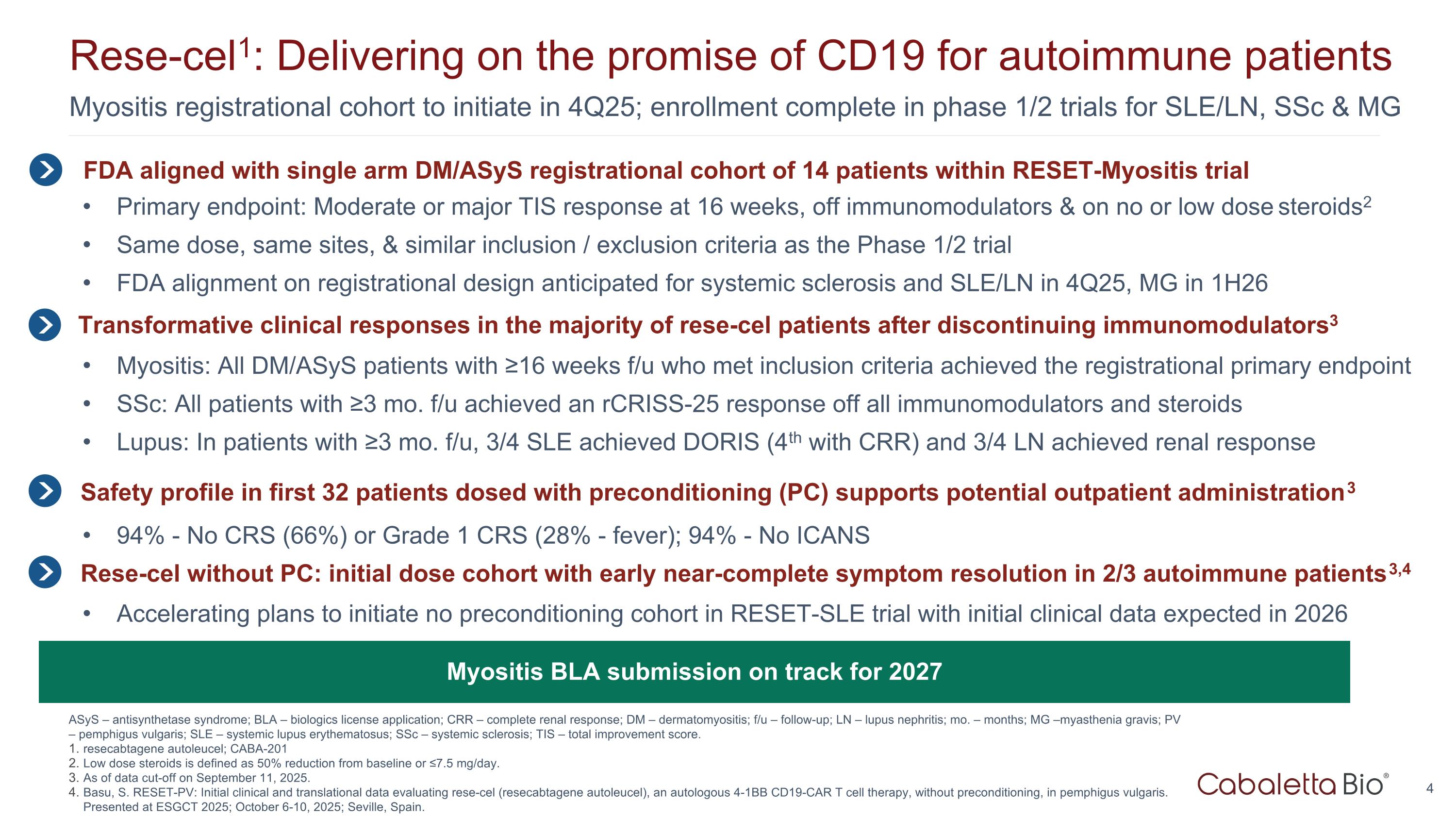
Myositis registrational cohort to initiate in 4Q25; enrollment complete in phase 1/2 trials for SLE/LN, SSc & MG Rese-cel1: Delivering on the promise of CD19 for autoimmune patients ASyS – antisynthetase syndrome; BLA – biologics license application; CRR – complete renal response; DM – dermatomyositis; f/u – follow-up; LN – lupus nephritis; mo. – months; MG –myasthenia gravis; PV – pemphigus vulgaris; SLE – systemic lupus erythematosus; SSc – systemic sclerosis; TIS – total improvement score. resecabtagene autoleucel; CABA-201 Low dose steroids is defined as 50% reduction from baseline or ≤7.5 mg/day. As of data cut-off on September 11, 2025. Basu, S. RESET-PV: Initial clinical and translational data evaluating rese-cel (resecabtagene autoleucel), an autologous 4-1BB CD19-CAR T cell therapy, without preconditioning, in pemphigus vulgaris. Presented at ESGCT 2025; October 6-10, 2025; Seville, Spain. FDA aligned with single arm DM/ASyS registrational cohort of 14 patients within RESET-Myositis trial Primary endpoint: Moderate or major TIS response at 16 weeks, off immunomodulators & on no or low dose steroids2 Same dose, same sites, & similar inclusion / exclusion criteria as the Phase 1/2 trial FDA alignment on registrational design anticipated for systemic sclerosis and SLE/LN in 4Q25, MG in 1H26 Myositis: All DM/ASyS patients with ≥16 weeks f/u who met inclusion criteria achieved the registrational primary endpoint SSc: All patients with ≥3 mo. f/u achieved an rCRISS-25 response off all immunomodulators and steroids Lupus: In patients with ≥3 mo. f/u, 3/4 SLE achieved DORIS (4th with CRR) and 3/4 LN achieved renal response Transformative clinical responses in the majority of rese-cel patients after discontinuing immunomodulators3 Myositis BLA submission on track for 2027 Rese-cel without PC: initial dose cohort with early near-complete symptom resolution in 2/3 autoimmune patients3,4 Safety profile in first 32 patients dosed with preconditioning (PC) supports potential outpatient administration3 94% - No CRS (66%) or Grade 1 CRS (28% - fever); 94% - No ICANS Accelerating plans to initiate no preconditioning cohort in RESET-SLE trial with initial clinical data expected in 2026
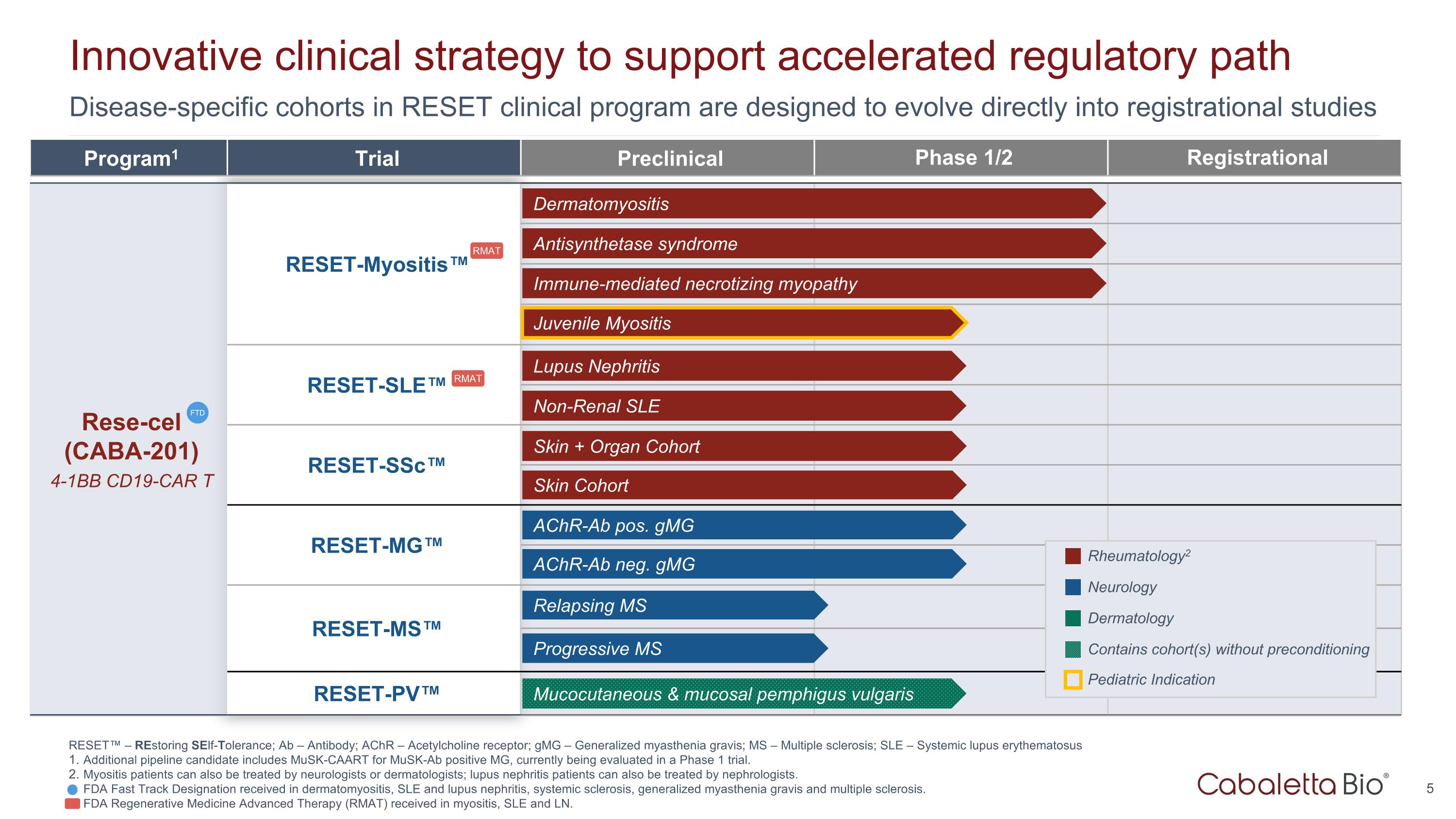
Disease-specific cohorts in RESET clinical program are designed to evolve directly into registrational studies Innovative clinical strategy to support accelerated regulatory path Program1 Trial Preclinical Phase 1/2 Registrational Rese-cel (CABA-201) 4-1BB CD19-CAR T RESET-Myositis™ CARTA Chimeric Antigen Receptor T cells for Autoimmunity RESET-SLE™ RESET-SSc™ RESET-MG™ RESET-MS™ RESET-PV™ Dermatomyositis Antisynthetase syndrome Immune-mediated necrotizing myopathy Lupus Nephritis Non-Renal SLE Skin + Organ Cohort AChR-Ab neg. gMG AChR-Ab pos. gMG Skin Cohort Rheumatology2 Neurology Dermatology FTD Mucocutaneous & mucosal pemphigus vulgaris Contains cohort(s) without preconditioning Juvenile Myositis Pediatric Indication RESET™ – REstoring SElf-Tolerance; Ab – Antibody; AChR – Acetylcholine receptor; gMG – Generalized myasthenia gravis; MS – Multiple sclerosis; SLE – Systemic lupus erythematosus Additional pipeline candidate includes MuSK-CAART for MuSK-Ab positive MG, currently being evaluated in a Phase 1 trial. Myositis patients can also be treated by neurologists or dermatologists; lupus nephritis patients can also be treated by nephrologists. FDA Fast Track Designation received in dermatomyositis, SLE and lupus nephritis, systemic sclerosis, generalized myasthenia gravis and multiple sclerosis. FDA Regenerative Medicine Advanced Therapy (RMAT) received in myositis, SLE and LN. Progressive MS Relapsing MS RMAT RMAT
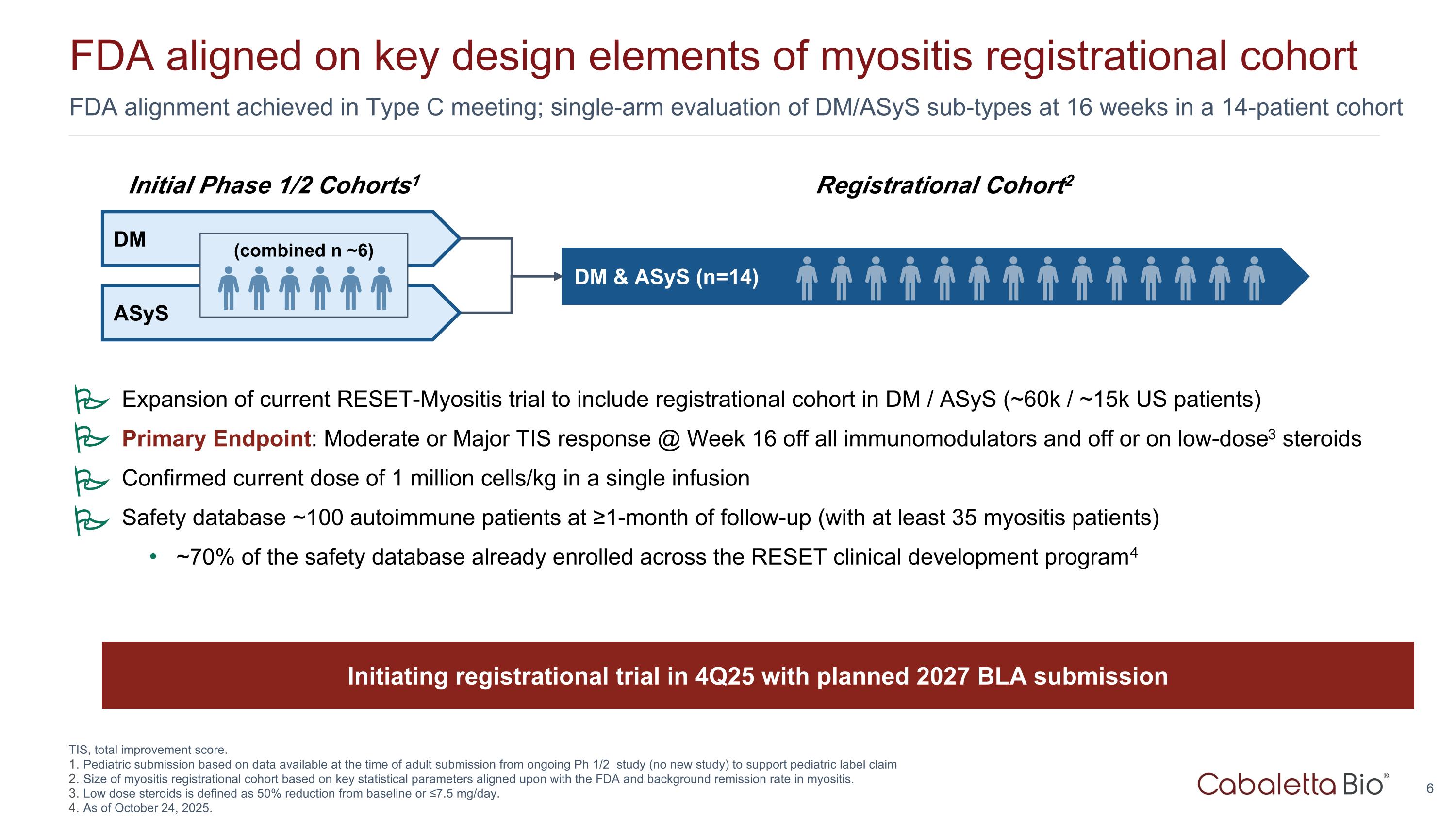
FDA alignment achieved in Type C meeting; single-arm evaluation of DM/ASyS sub-types at 16 weeks in a 14-patient cohort FDA aligned on key design elements of myositis registrational cohort Registrational Cohort2 ASyS DM & ASyS (n=14) Initial Phase 1/2 Cohorts1 DM (combined n ~6) TIS, total improvement score. Pediatric submission based on data available at the time of adult submission from ongoing Ph 1/2 study (no new study) to support pediatric label claim Size of myositis registrational cohort based on key statistical parameters aligned upon with the FDA and background remission rate in myositis. Low dose steroids is defined as 50% reduction from baseline or ≤7.5 mg/day. As of October 24, 2025. Initiating registrational trial in 4Q25 with planned 2027 BLA submission Expansion of current RESET-Myositis trial to include registrational cohort in DM / ASyS (~60k / ~15k US patients) Primary Endpoint: Moderate or Major TIS response @ Week 16 off all immunomodulators and off or on low-dose3 steroids Confirmed current dose of 1 million cells/kg in a single infusion Safety database ~100 autoimmune patients at ≥1-month of follow-up (with at least 35 myositis patients) ~70% of the safety database already enrolled across the RESET clinical development program4 P P P P
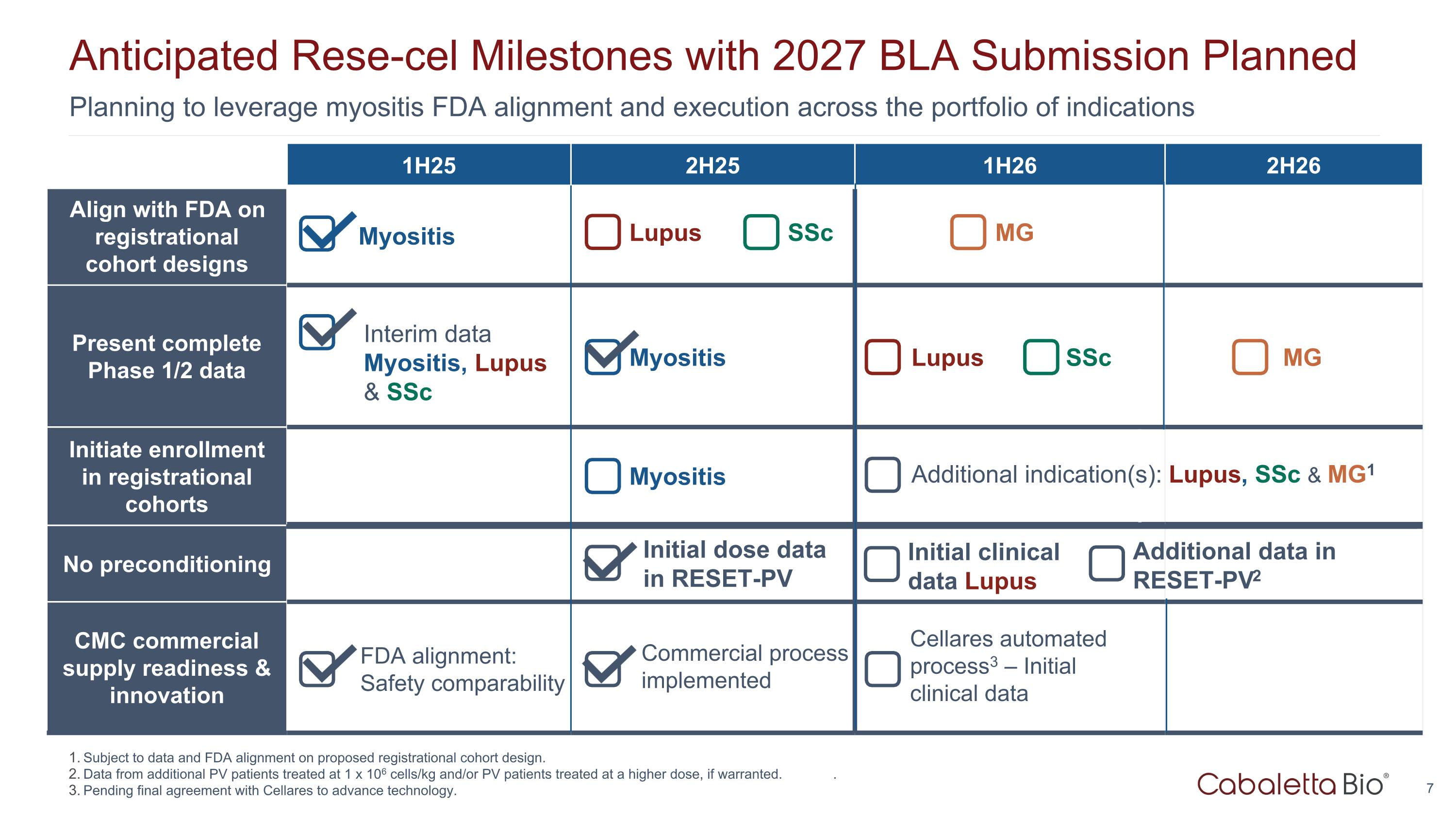
Planning to leverage myositis FDA alignment and execution across the portfolio of indications Anticipated Rese-cel Milestones with 2027 BLA Submission Planned 1H25 2H25 1H26 2H26 Align with FDA on registrational cohort designs Present complete Phase 1/2 data Initiate enrollment in registrational cohorts No preconditioning CMC commercial supply readiness & innovation Initial clinical data Lupus Initial dose data in RESET-PV Commercial process implemented Lupus SSc Myositis MG Lupus SSc MG Myositis Myositis FDA alignment: Safety comparability Interim data Myositis, Lupus & SSc Cellares automated process3 – Initial clinical data Subject to data and FDA alignment on proposed registrational cohort design. Data from additional PV patients treated at 1 x 106 cells/kg and/or PV patients treated at a higher dose, if warranted. . Pending final agreement with Cellares to advance technology. Additional indication(s): Lupus, SSc & MG1 Additional data in RESET-PV2

Rese-cel: Autologus CD19-CAR T for Autoimmunity
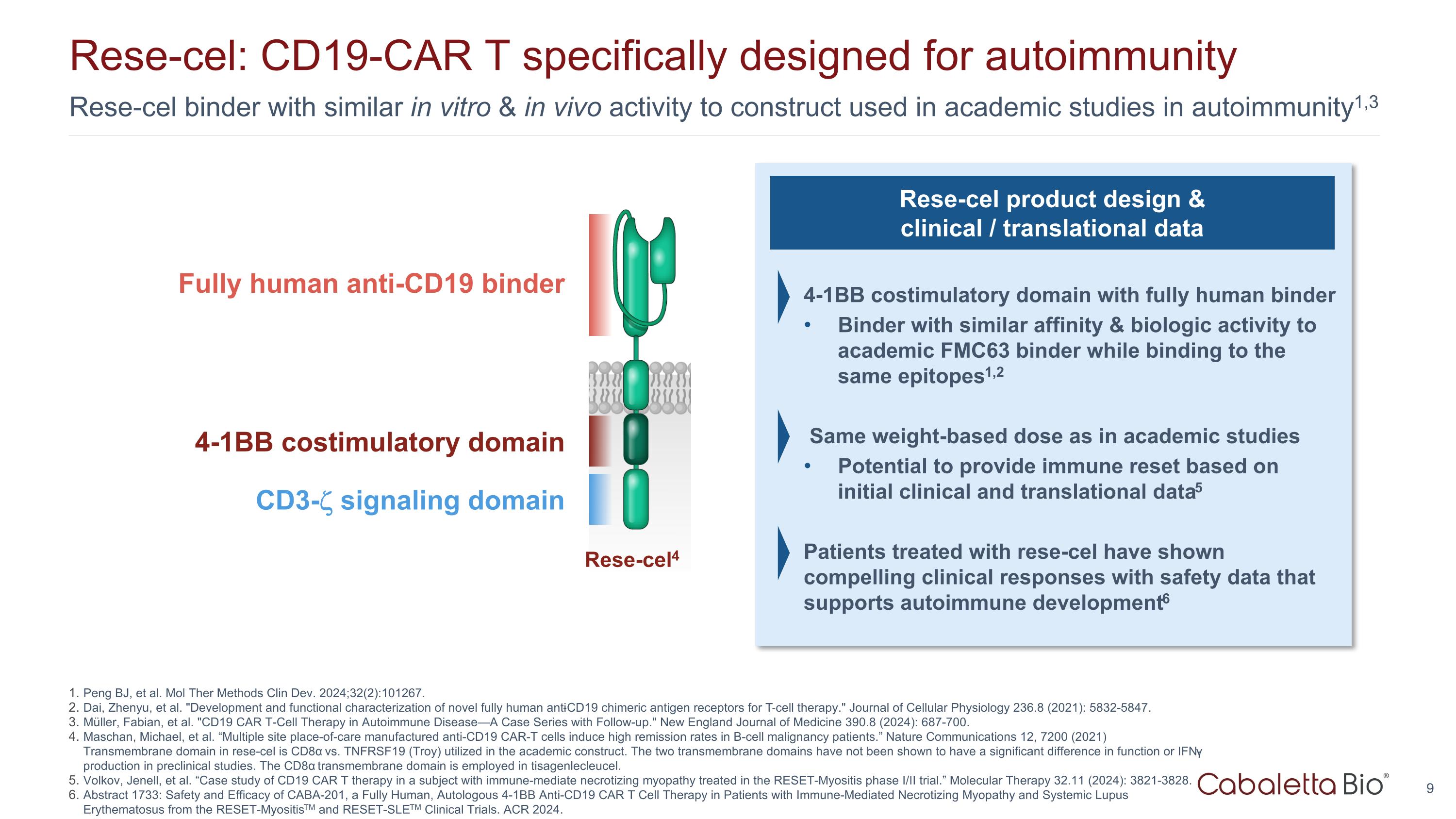
Rese-cel binder with similar in vitro & in vivo activity to construct used in academic studies in autoimmunity1,3 Rese-cel: CD19-CAR T specifically designed for autoimmunity Peng BJ, et al. Mol Ther Methods Clin Dev. 2024;32(2):101267. Dai, Zhenyu, et al. "Development and functional characterization of novel fully human anti‐CD19 chimeric antigen receptors for T‐cell therapy." Journal of Cellular Physiology 236.8 (2021): 5832-5847. Müller, Fabian, et al. "CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up." New England Journal of Medicine 390.8 (2024): 687-700. Maschan, Michael, et al. “Multiple site place-of-care manufactured anti-CD19 CAR-T cells induce high remission rates in B-cell malignancy patients.” Nature Communications 12, 7200 (2021) Transmembrane domain in rese-cel is CD8α vs. TNFRSF19 (Troy) utilized in the academic construct. The two transmembrane domains have not been shown to have a significant difference in function or IFN-γ production in preclinical studies. The CD8α transmembrane domain is employed in tisagenlecleucel. Volkov, Jenell, et al. “Case study of CD19 CAR T therapy in a subject with immune-mediate necrotizing myopathy treated in the RESET-Myositis phase I/II trial.” Molecular Therapy 32.11 (2024): 3821-3828. Abstract 1733: Safety and Efficacy of CABA-201, a Fully Human, Autologous 4-1BB Anti-CD19 CAR T Cell Therapy in Patients with Immune-Mediated Necrotizing Myopathy and Systemic Lupus Erythematosus from the RESET-MyositisTM and RESET-SLETM Clinical Trials. ACR 2024. Rese-cel product design & clinical / translational data 4-1BB costimulatory domain with fully human binder Binder with similar affinity & biologic activity to academic FMC63 binder while binding to the same epitopes1,2 Same weight-based dose as in academic studies Potential to provide immune reset based on initial clinical and translational data5 Patients treated with rese-cel have shown compelling clinical responses with safety data that supports autoimmune development6 Fully human anti-CD19 binder 4-1BB costimulatory domain CD3- signaling domain Rese-cel4
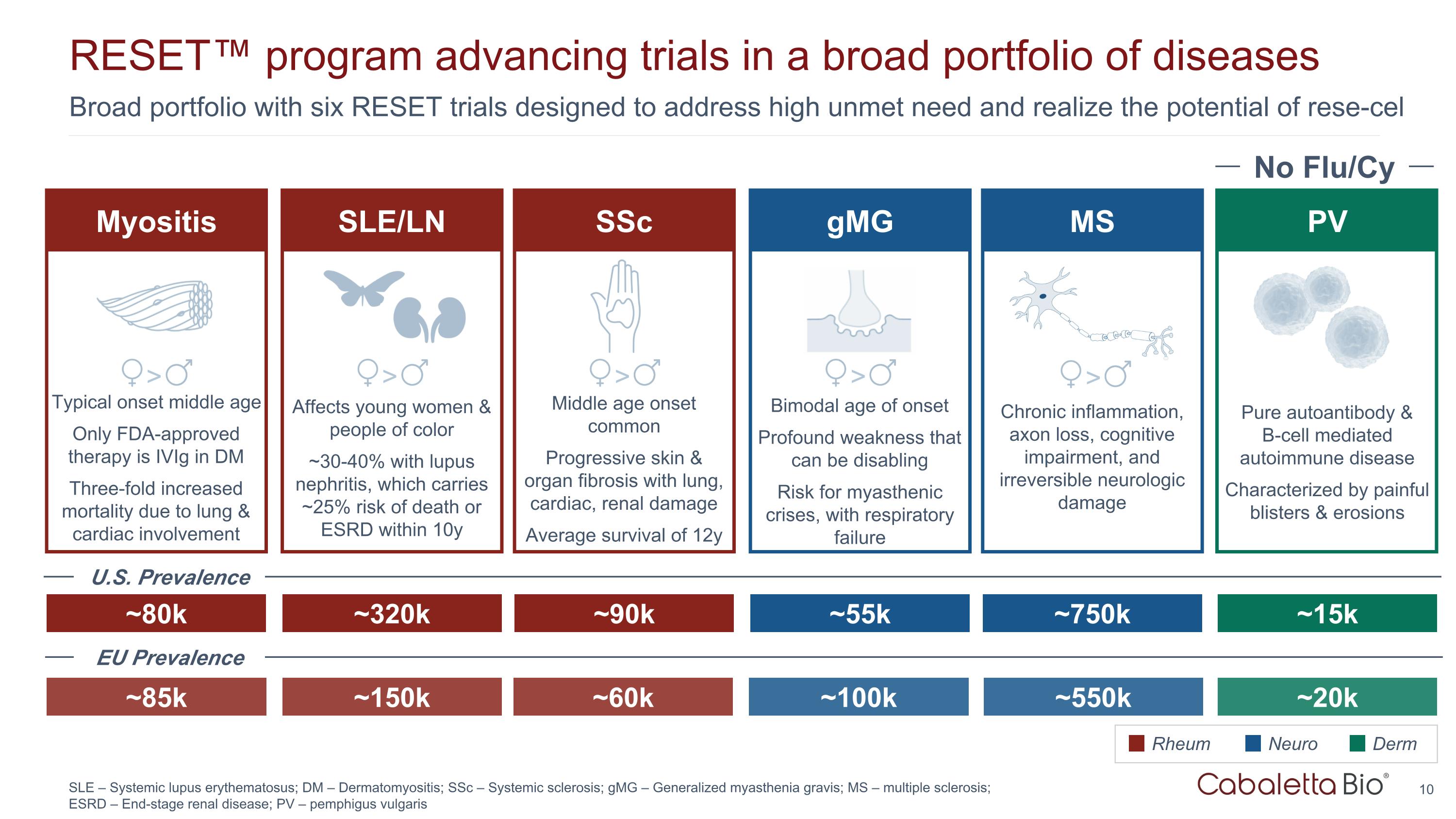
Broad portfolio with six RESET trials designed to address high unmet need and realize the potential of rese-cel RESET™ program advancing trials in a broad portfolio of diseases SLE – Systemic lupus erythematosus; DM – Dermatomyositis; SSc – Systemic sclerosis; gMG – Generalized myasthenia gravis; MS – multiple sclerosis; ESRD – End-stage renal disease; PV – pemphigus vulgaris SSc gMG PV ~90k ~55k ~15k Middle age onset common Progressive skin & organ fibrosis with lung, cardiac, renal damage Average survival of 12y Bimodal age of onset Profound weakness that can be disabling Risk for myasthenic crises, with respiratory failure Rheum Neuro Derm No Flu/Cy Pure autoantibody & B-cell mediated autoimmune disease Characterized by painful blisters & erosions SLE/LN ~320k Affects young women & people of color ~30-40% with lupus nephritis, which carries ~25% risk of death or ESRD within 10y > > > Myositis ~80k Typical onset middle age Only FDA-approved therapy is IVIg in DM Three-fold increased mortality due to lung & cardiac involvement > MS ~750k Chronic inflammation, axon loss, cognitive impairment, and irreversible neurologic damage > ~85k ~150k ~60k ~100k ~550k ~20k U.S. Prevalence EU Prevalence
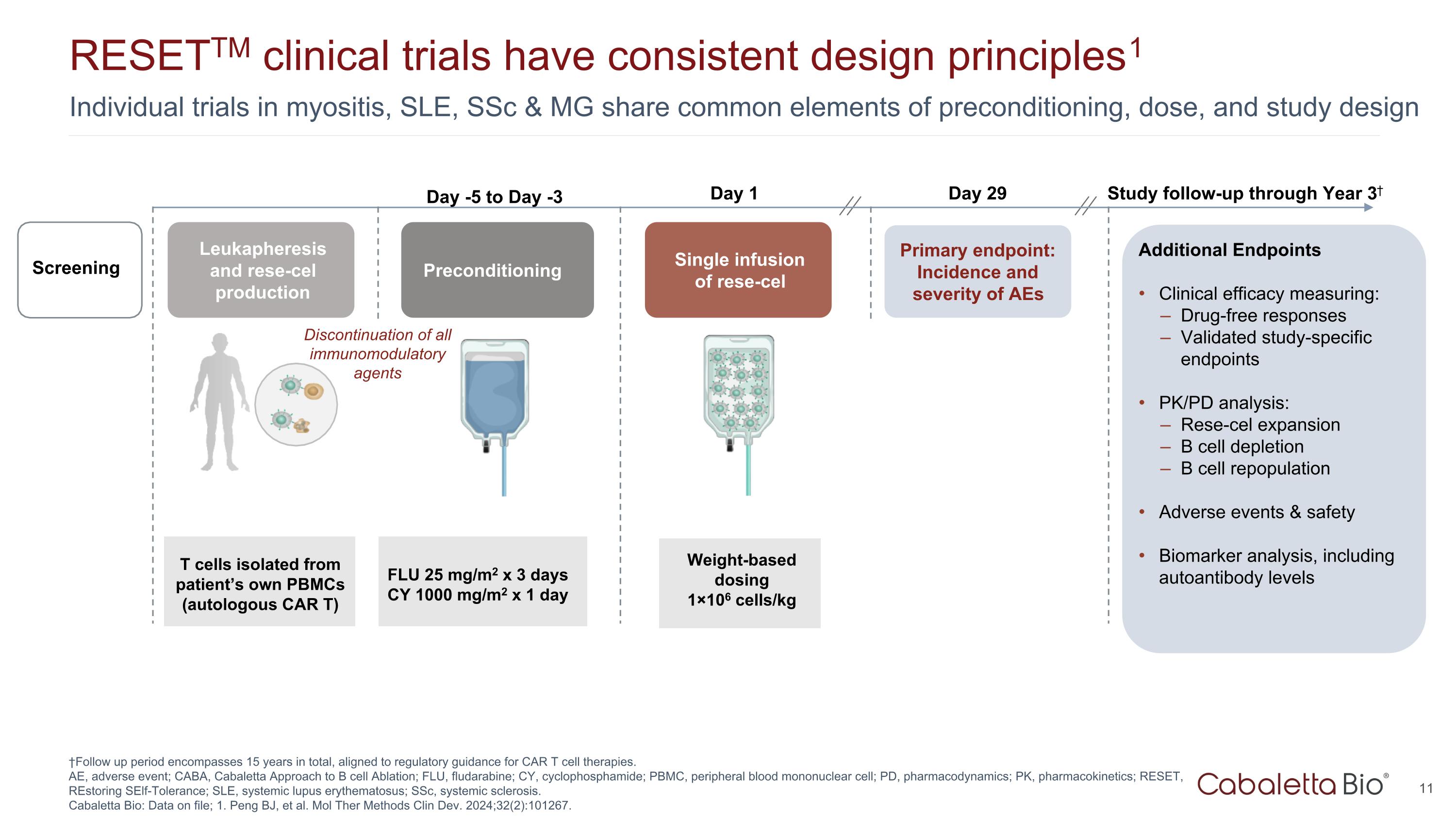
Individual trials in myositis, SLE, SSc & MG share common elements of preconditioning, dose, and study design RESETTM clinical trials have consistent design principles1 †Follow up period encompasses 15 years in total, aligned to regulatory guidance for CAR T cell therapies. AE, adverse event; CABA, Cabaletta Approach to B cell Ablation; FLU, fludarabine; CY, cyclophosphamide; PBMC, peripheral blood mononuclear cell; PD, pharmacodynamics; PK, pharmacokinetics; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus; SSc, systemic sclerosis. Cabaletta Bio: Data on file; 1. Peng BJ, et al. Mol Ther Methods Clin Dev. 2024;32(2):101267. Leukapheresis and rese-cel production Preconditioning Single infusion of rese-cel Weight-based dosing 1×106 cells/kg Day 1 Primary endpoint: Incidence and severity of AEs T cells isolated from patient’s own PBMCs (autologous CAR T) Day 29 Study follow-up through Year 3† Screening Additional Endpoints Clinical efficacy measuring: Drug-free responses Validated study-specific endpoints PK/PD analysis: Rese-cel expansion B cell depletion B cell repopulation Adverse events & safety Biomarker analysis, including autoantibody levels FLU 25 mg/m2 x 3 days CY 1000 mg/m2 x 1 day Day -5 to Day -3 Discontinuation of all immunomodulatory agents
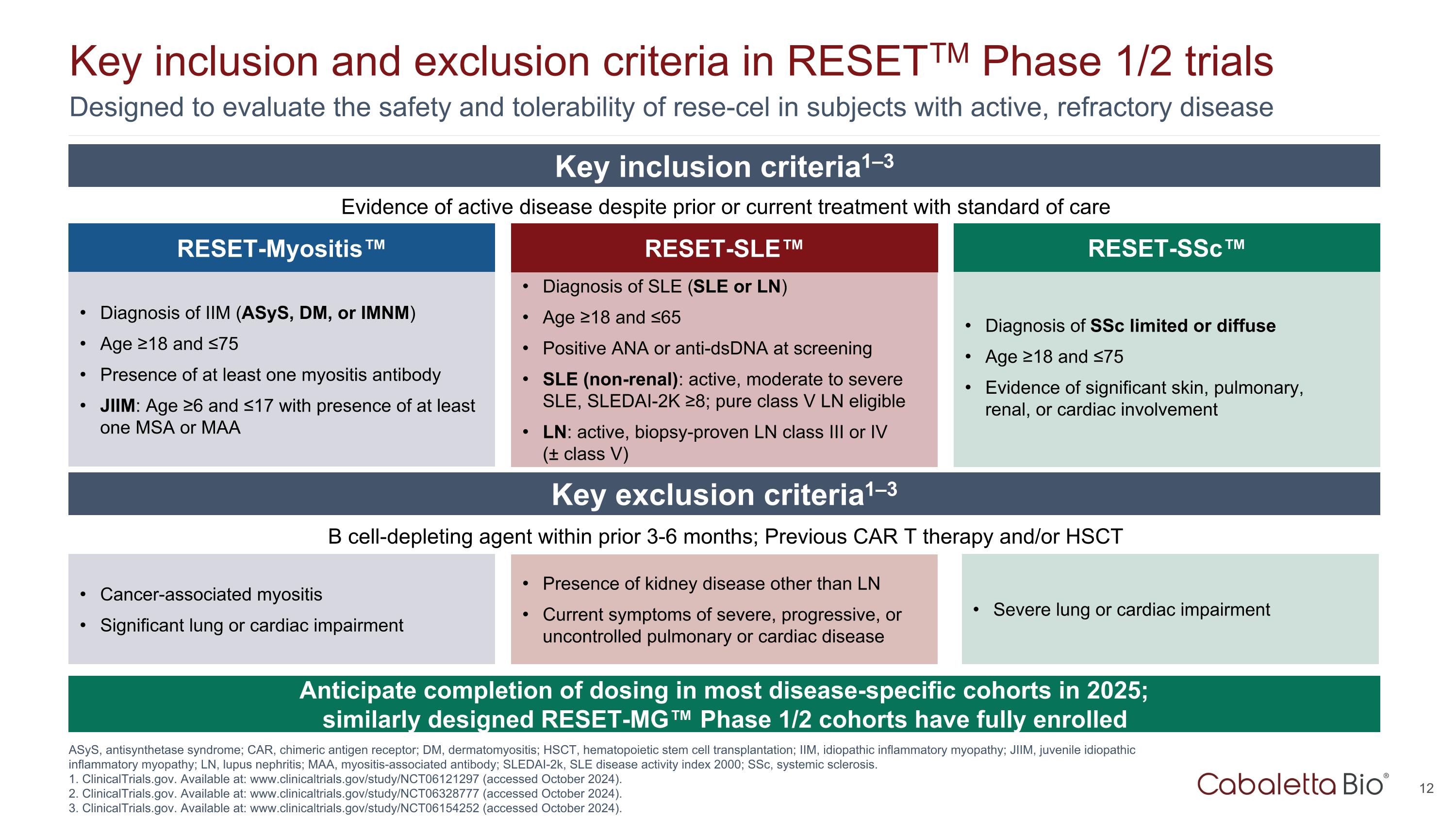
Designed to evaluate the safety and tolerability of rese-cel in subjects with active, refractory disease Key inclusion and exclusion criteria in RESETTM Phase 1/2 trials ASyS, antisynthetase syndrome; CAR, chimeric antigen receptor; DM, dermatomyositis; HSCT, hematopoietic stem cell transplantation; IIM, idiopathic inflammatory myopathy; JIIM, juvenile idiopathic inflammatory myopathy; LN, lupus nephritis; MAA, myositis-associated antibody; SLEDAI-2k, SLE disease activity index 2000; SSc, systemic sclerosis. 1. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06121297 (accessed October 2024). 2. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06328777 (accessed October 2024). 3. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06154252 (accessed October 2024). RESET-SLE™ Diagnosis of SLE (SLE or LN) Age ≥18 and ≤65 Positive ANA or anti-dsDNA at screening SLE (non-renal): active, moderate to severe SLE, SLEDAI-2K ≥8; pure class V LN eligible LN: active, biopsy-proven LN class III or IV (± class V) RESET-Myositis™ Diagnosis of IIM (ASyS, DM, or IMNM) Age ≥18 and ≤75 Presence of at least one myositis antibody JIIM: Age ≥6 and ≤17 with presence of at least one MSA or MAA Diagnosis of SSc limited or diffuse Age ≥18 and ≤75 Evidence of significant skin, pulmonary, renal, or cardiac involvement RESET-SSc™ Evidence of active disease despite prior or current treatment with standard of care Key inclusion criteria1–3 Key exclusion criteria1–3 B cell-depleting agent within prior 3-6 months; Previous CAR T therapy and/or HSCT Presence of kidney disease other than LN Current symptoms of severe, progressive, or uncontrolled pulmonary or cardiac disease Cancer-associated myositis Significant lung or cardiac impairment Severe lung or cardiac impairment Anticipate completion of dosing in most disease-specific cohorts in 2025; similarly designed RESET-MG™ Phase 1/2 cohorts have fully enrolled
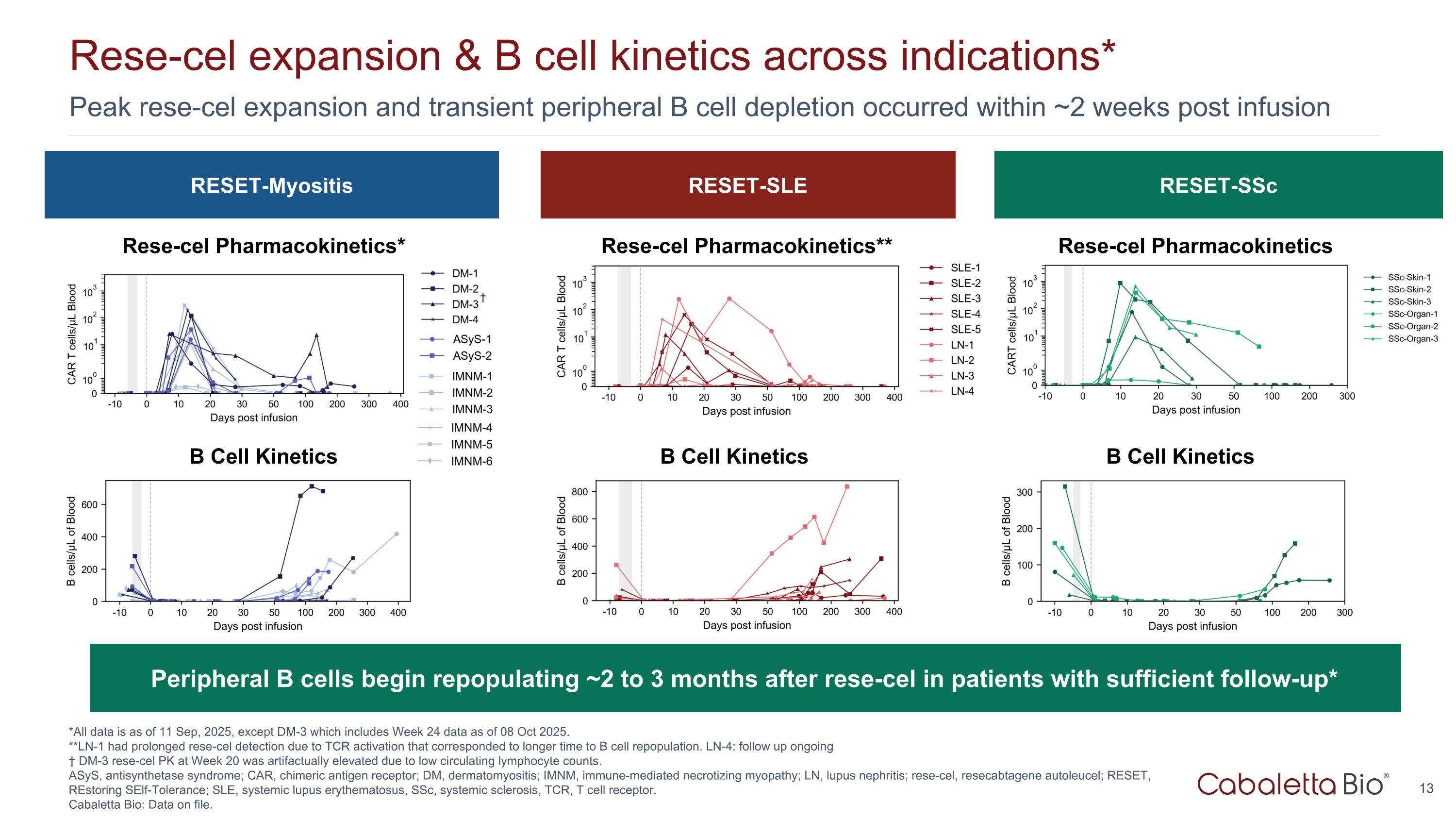
Peak rese-cel expansion and transient peripheral B cell depletion occurred within ~2 weeks post infusion Rese-cel expansion & B cell kinetics across indications* *All data is as of 11 Sep, 2025, except DM-3 which includes Week 24 data as of 08 Oct 2025. **LN-1 had prolonged rese-cel detection due to TCR activation that corresponded to longer time to B cell repopulation. LN-4: follow up ongoing † DM-3 rese-cel PK at Week 20 was artifactually elevated due to low circulating lymphocyte counts. ASyS, antisynthetase syndrome; CAR, chimeric antigen receptor; DM, dermatomyositis; IMNM, immune-mediated necrotizing myopathy; LN, lupus nephritis; rese-cel, resecabtagene autoleucel; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus, SSc, systemic sclerosis, TCR, T cell receptor. Cabaletta Bio: Data on file. RESET-Myositis RESET-SLE RESET-SSc Rese-cel Pharmacokinetics* Rese-cel Pharmacokinetics** Rese-cel Pharmacokinetics B Cell Kinetics B Cell Kinetics B Cell Kinetics Peripheral B cells begin repopulating ~2 to 3 months after rese-cel in patients with sufficient follow-up* †

Myositis: Unmet Need & Clinical Data
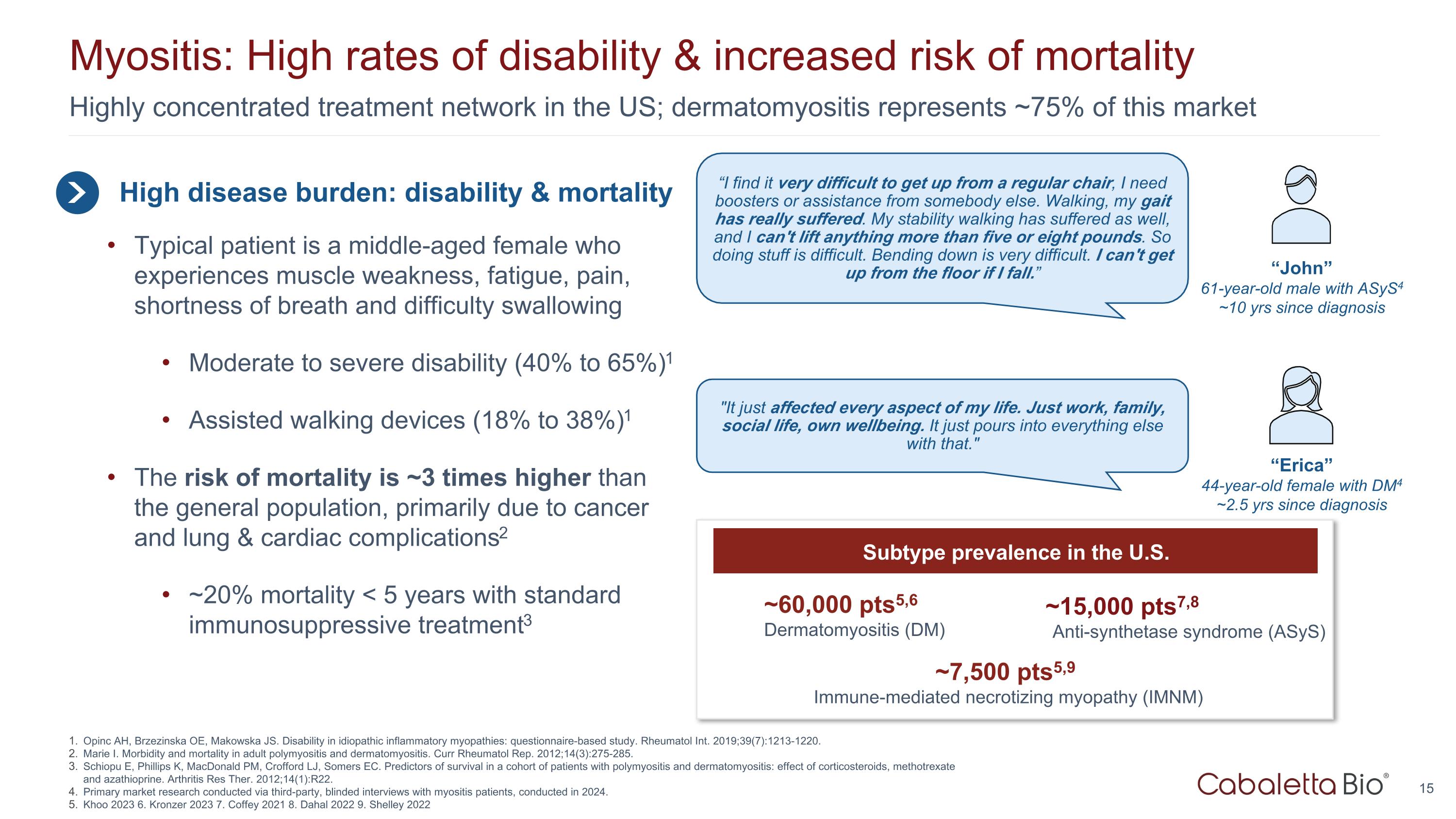
Highly concentrated treatment network in the US; dermatomyositis represents ~75% of this market Myositis: High rates of disability & increased risk of mortality High disease burden: disability & mortality Typical patient is a middle-aged female who experiences muscle weakness, fatigue, pain, shortness of breath and difficulty swallowing Moderate to severe disability (40% to 65%)1 Assisted walking devices (18% to 38%)1 The risk of mortality is ~3 times higher than the general population, primarily due to cancer and lung & cardiac complications2 ~20% mortality < 5 years with standard immunosuppressive treatment3 “I find it very difficult to get up from a regular chair, I need boosters or assistance from somebody else. Walking, my gait has really suffered. My stability walking has suffered as well, and I can't lift anything more than five or eight pounds. So doing stuff is difficult. Bending down is very difficult. I can't get up from the floor if I fall.” “John” 61-year-old male with ASyS4 ~10 yrs since diagnosis "It just affected every aspect of my life. Just work, family, social life, own wellbeing. It just pours into everything else with that." “Erica” 44-year-old female with DM4 ~2.5 yrs since diagnosis Opinc AH, Brzezinska OE, Makowska JS. Disability in idiopathic inflammatory myopathies: questionnaire-based study. Rheumatol Int. 2019;39(7):1213-1220. Marie I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep. 2012;14(3):275-285. Schiopu E, Phillips K, MacDonald PM, Crofford LJ, Somers EC. Predictors of survival in a cohort of patients with polymyositis and dermatomyositis: effect of corticosteroids, methotrexate and azathioprine. Arthritis Res Ther. 2012;14(1):R22. Primary market research conducted via third-party, blinded interviews with myositis patients, conducted in 2024. Khoo 2023 6. Kronzer 2023 7. Coffey 2021 8. Dahal 2022 9. Shelley 2022 ~7,500 pts5,9 Immune-mediated necrotizing myopathy (IMNM) ~15,000 pts7,8 Anti-synthetase syndrome (ASyS) ~60,000 pts5,6 Dermatomyositis (DM) Subtype prevalence in the U.S.
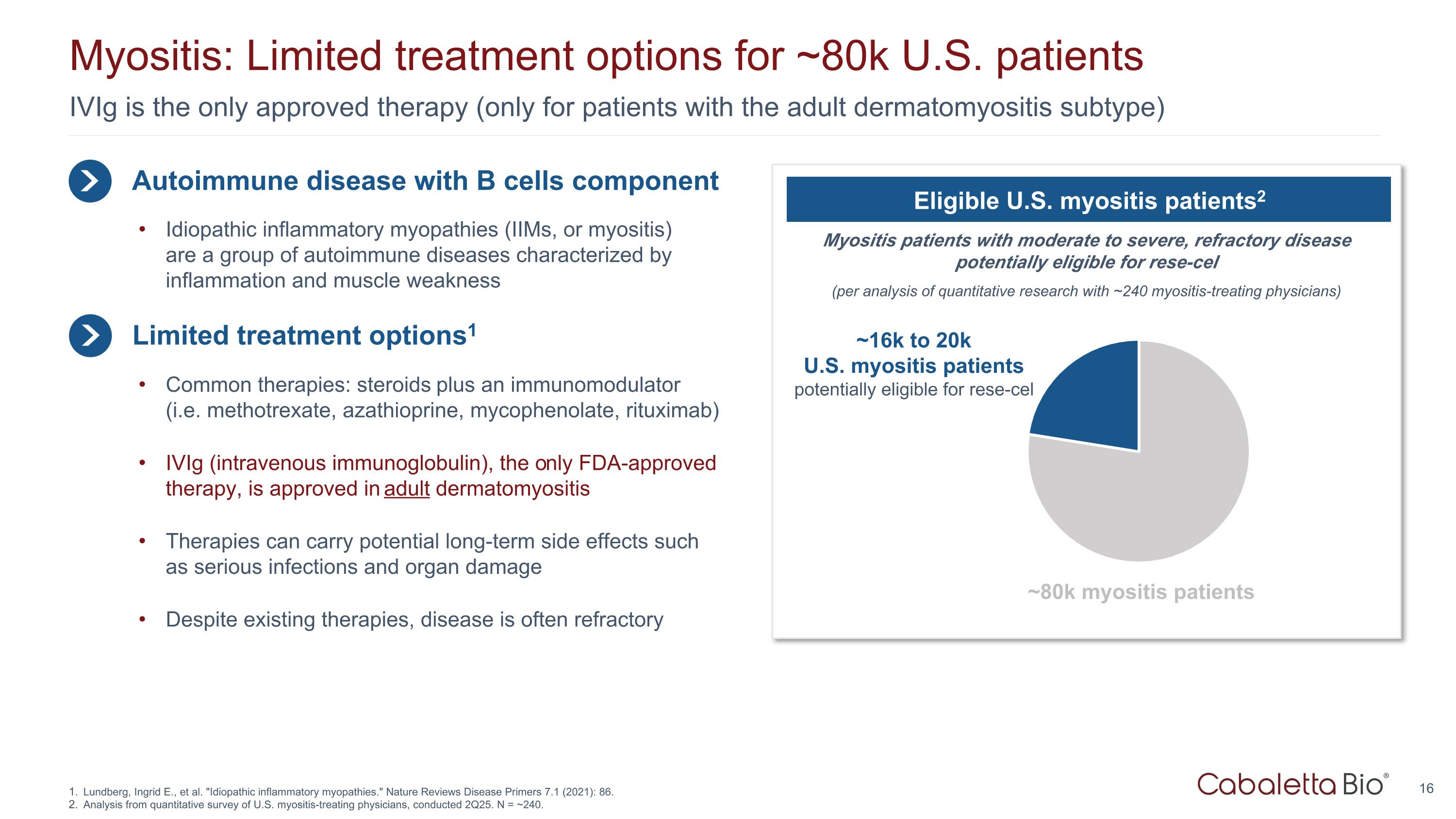
IVIg is the only approved therapy (only for patients with the adult dermatomyositis subtype) Myositis: Limited treatment options for ~80k U.S. patients Lundberg, Ingrid E., et al. "Idiopathic inflammatory myopathies." Nature Reviews Disease Primers 7.1 (2021): 86. Analysis from quantitative survey of U.S. myositis-treating physicians, conducted 2Q25. N = ~240. Autoimmune disease with B cells component Limited treatment options1 Idiopathic inflammatory myopathies (IIMs, or myositis) are a group of autoimmune diseases characterized by inflammation and muscle weakness Common therapies: steroids plus an immunomodulator (i.e. methotrexate, azathioprine, mycophenolate, rituximab) IVIg (intravenous immunoglobulin), the only FDA-approved therapy, is approved in adult dermatomyositis Therapies can carry potential long-term side effects such as serious infections and organ damage Despite existing therapies, disease is often refractory Myositis patients with moderate to severe, refractory disease potentially eligible for rese-cel (per analysis of quantitative research with ~240 myositis-treating physicians) Eligible U.S. myositis patients2 ~16k to 20k U.S. myositis patients potentially eligible for rese-cel ~80k myositis patients
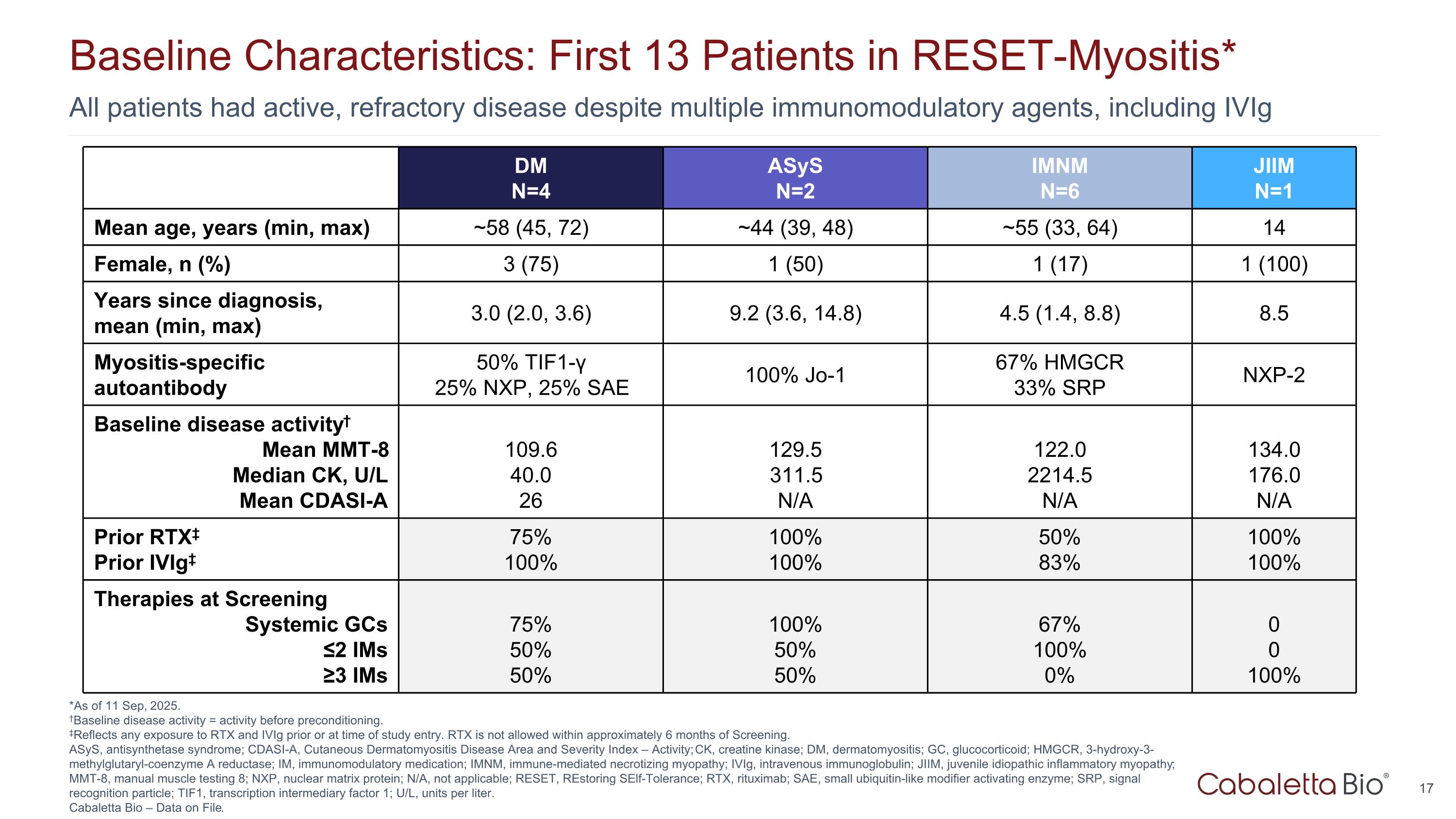
All patients had active, refractory disease despite multiple immunomodulatory agents, including IVIg Baseline Characteristics: First 13 Patients in RESET-Myositis* *As of 11 Sep, 2025. †Baseline disease activity = activity before preconditioning. ‡Reflects any exposure to RTX and IVIg prior or at time of study entry. RTX is not allowed within approximately 6 months of Screening. ASyS, antisynthetase syndrome; CDASI-A, Cutaneous Dermatomyositis Disease Area and Severity Index – Activity; CK, creatine kinase; DM, dermatomyositis; GC, glucocorticoid; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IM, immunomodulatory medication; IMNM, immune-mediated necrotizing myopathy; IVIg, intravenous immunoglobulin; JIIM, juvenile idiopathic inflammatory myopathy; MMT-8, manual muscle testing 8; NXP, nuclear matrix protein; N/A, not applicable; RESET, REstoring SElf-Tolerance; RTX, rituximab; SAE, small ubiquitin-like modifier activating enzyme; SRP, signal recognition particle; TIF1, transcription intermediary factor 1; U/L, units per liter. Cabaletta Bio – Data on File. DM N=4 ASyS N=2 IMNM N=6 JIIM N=1 Mean age, years (min, max) ~58 (45, 72) ~44 (39, 48) ~55 (33, 64) 14 Female, n (%) 3 (75) 1 (50) 1 (17) 1 (100) Years since diagnosis, mean (min, max) 3.0 (2.0, 3.6) 9.2 (3.6, 14.8) 4.5 (1.4, 8.8) 8.5 Myositis-specific autoantibody 50% TIF1-γ 25% NXP, 25% SAE 100% Jo-1 67% HMGCR 33% SRP NXP-2 Baseline disease activity† Mean MMT-8 Median CK, U/L Mean CDASI-A 109.6 40.0 26 129.5 311.5 N/A 122.0 2214.5 N/A 134.0 176.0 N/A Prior RTX‡ Prior IVIg‡ 75% 100% 100% 100% 50% 83% 100% 100% Therapies at Screening Systemic GCs ≤2 IMs ≥3 IMs 75% 50% 50% 100% 50% 50% 67% 100% 0% 0 0 100%
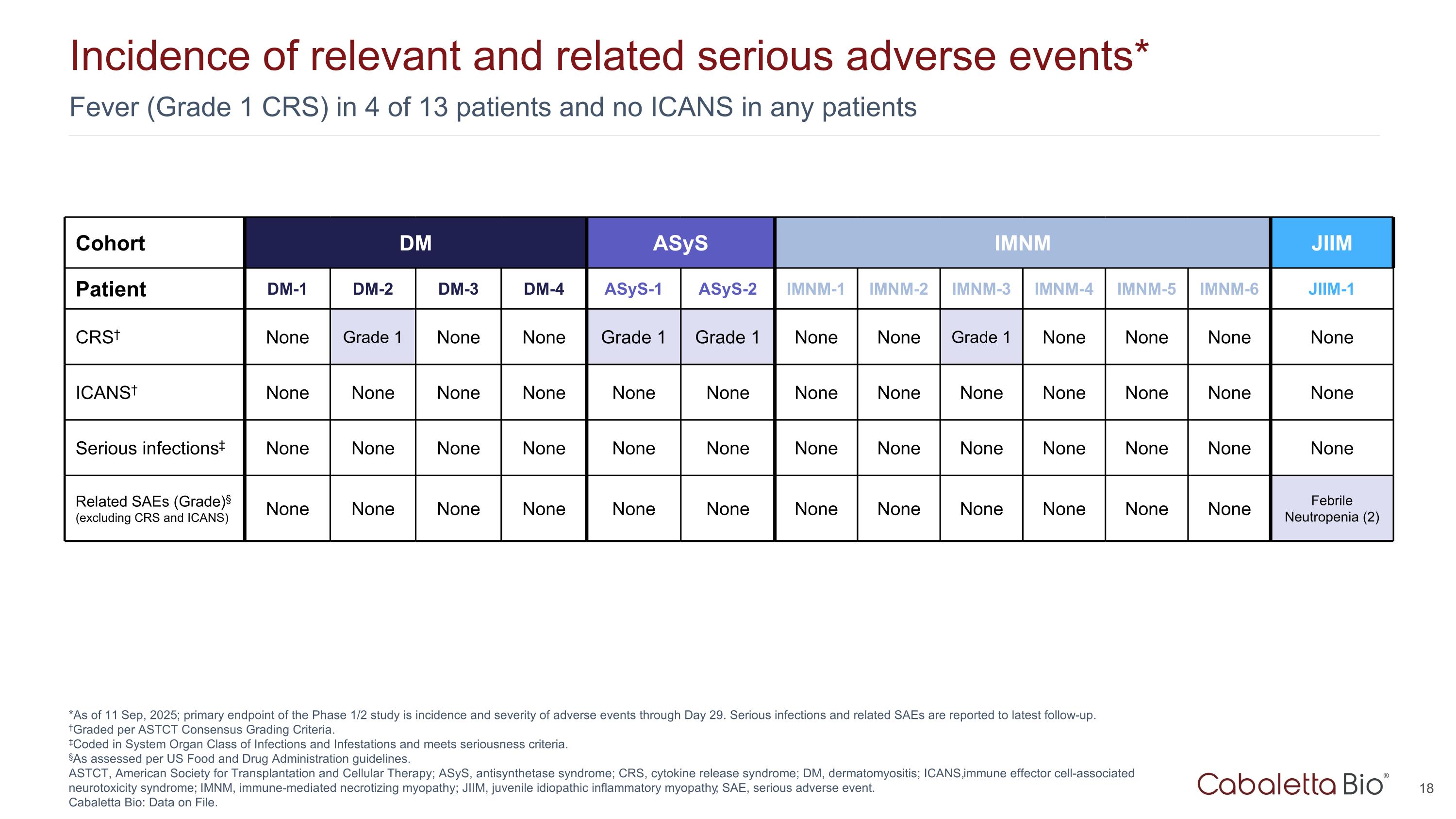
Fever (Grade 1 CRS) in 4 of 13 patients and no ICANS in any patients Incidence of relevant and related serious adverse events* *As of 11 Sep, 2025; primary endpoint of the Phase 1/2 study is incidence and severity of adverse events through Day 29. Serious infections and related SAEs are reported to latest follow-up. †Graded per ASTCT Consensus Grading Criteria. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per US Food and Drug Administration guidelines. ASTCT, American Society for Transplantation and Cellular Therapy; ASyS, antisynthetase syndrome; CRS, cytokine release syndrome; DM, dermatomyositis; ICANS, immune effector cell-associated neurotoxicity syndrome; IMNM, immune-mediated necrotizing myopathy; JIIM, juvenile idiopathic inflammatory myopathy; SAE, serious adverse event. Cabaletta Bio: Data on File. Cohort DM ASyS IMNM JIIM Patient DM-1 DM-2 DM-3 DM-4 ASyS-1 ASyS-2 IMNM-1 IMNM-2 IMNM-3 IMNM-4 IMNM-5 IMNM-6 JIIM-1 CRS† None Grade 1 None None Grade 1 Grade 1 None None Grade 1 None None None None ICANS† None None None None None None None None None None None None None Serious infections‡ None None None None None None None None None None None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None None None None None None None None None None Febrile Neutropenia (2)
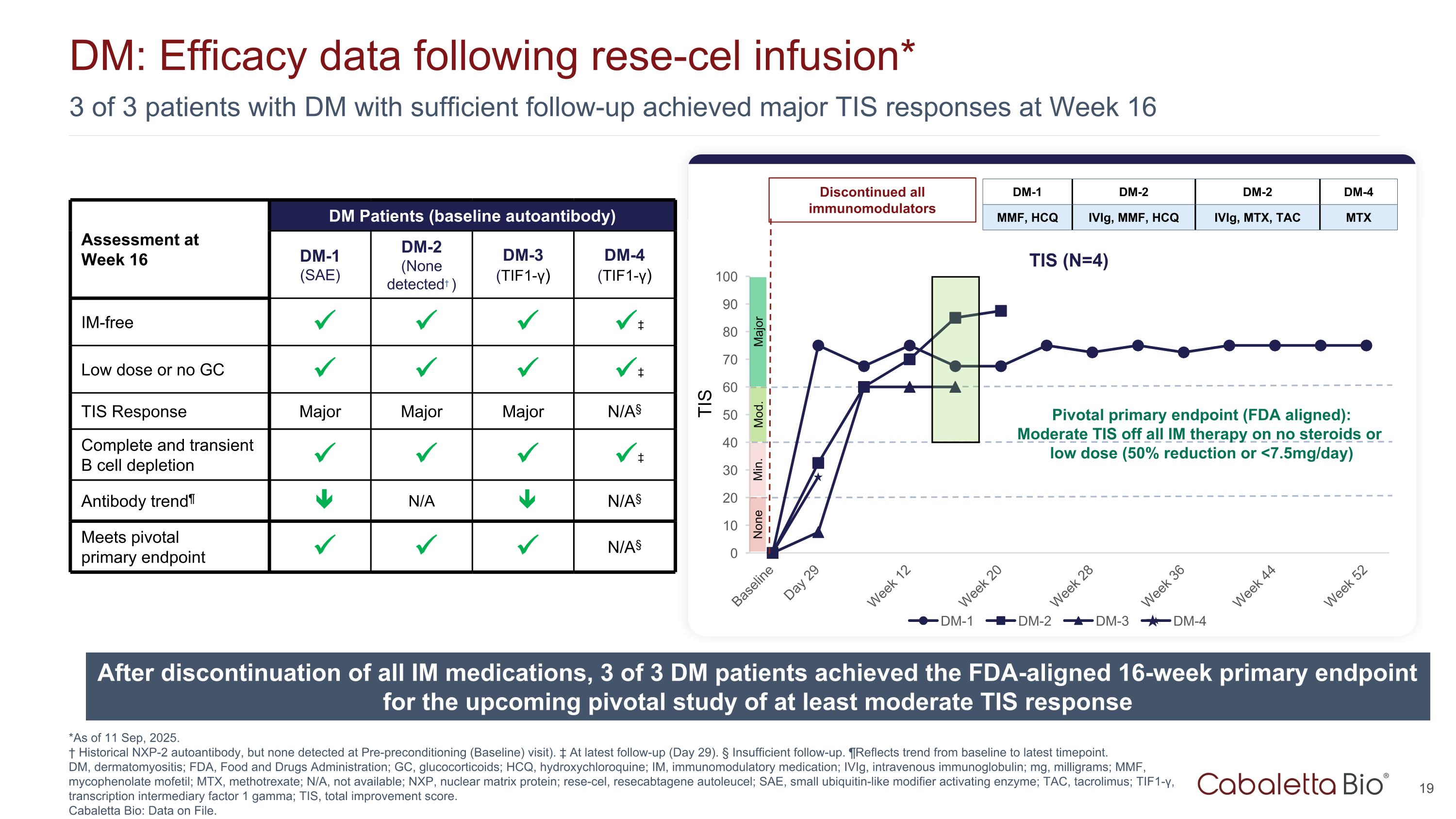
3 of 3 patients with DM with sufficient follow-up achieved major TIS responses at Week 16 DM: Efficacy data following rese-cel infusion* *As of 11 Sep, 2025. † Historical NXP-2 autoantibody, but none detected at Pre-preconditioning (Baseline) visit). ‡ At latest follow-up (Day 29). § Insufficient follow-up. ¶Reflects trend from baseline to latest timepoint. DM, dermatomyositis; FDA, Food and Drugs Administration; GC, glucocorticoids; HCQ, hydroxychloroquine; IM, immunomodulatory medication; IVIg, intravenous immunoglobulin; mg, milligrams; MMF, mycophenolate mofetil; MTX, methotrexate; N/A, not available; NXP, nuclear matrix protein; rese-cel, resecabtagene autoleucel; SAE, small ubiquitin-like modifier activating enzyme; TAC, tacrolimus; TIF1-γ, transcription intermediary factor 1 gamma; TIS, total improvement score. Cabaletta Bio: Data on File. After discontinuation of all IM medications, 3 of 3 DM patients achieved the FDA-aligned 16-week primary endpoint for the upcoming pivotal study of at least moderate TIS response TIS Mod. Major Min. None TIS Discontinued all immunomodulators DM-1 DM-2 DM-2 DM-4 MMF, HCQ IVIg, MMF, HCQ IVIg, MTX, TAC MTX Pivotal primary endpoint (FDA aligned): Moderate TIS off all IM therapy on no steroids or low dose (50% reduction or <7.5mg/day) Assessment at Week 16 DM Patients (baseline autoantibody) DM-1 (SAE) DM-2 (None detected† ) DM-3 (TIF1-γ) DM-4 (TIF1-γ) IM-free ‡ Low dose or no GC ‡ TIS Response Major Major Major N/A§ Complete and transient B cell depletion ‡ Antibody trend¶ N/A N/A§ Meets pivotal primary endpoint N/A§ TIS (N=4)
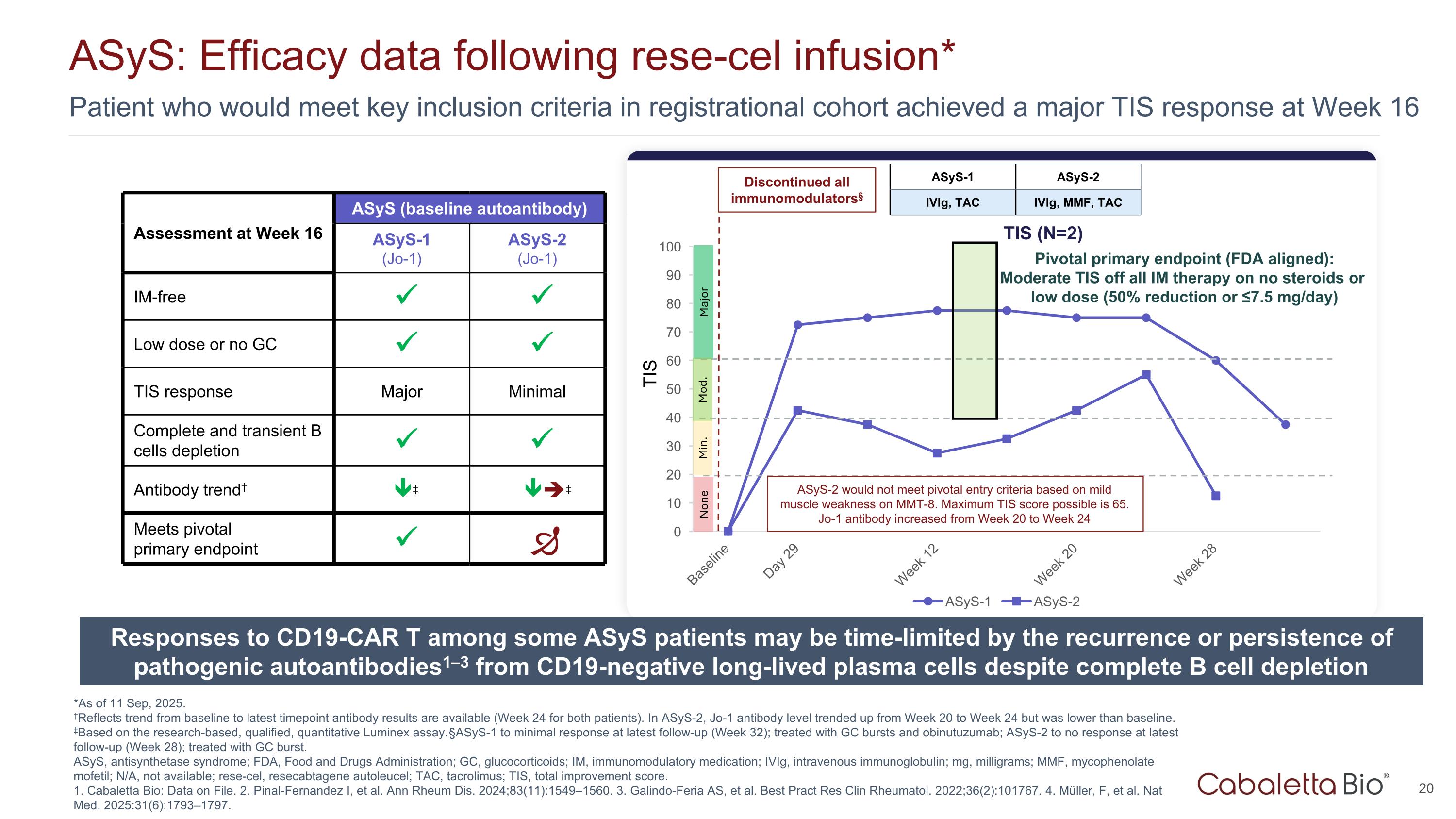
Patient who would meet key inclusion criteria in registrational cohort achieved a major TIS response at Week 16 ASyS: Efficacy data following rese-cel infusion* *As of 11 Sep, 2025. †Reflects trend from baseline to latest timepoint antibody results are available (Week 24 for both patients). In ASyS-2, Jo-1 antibody level trended up from Week 20 to Week 24 but was lower than baseline. ‡Based on the research-based, qualified, quantitative Luminex assay. §ASyS-1 to minimal response at latest follow-up (Week 32); treated with GC bursts and obinutuzumab; ASyS-2 to no response at latest follow-up (Week 28); treated with GC burst. ASyS, antisynthetase syndrome; FDA, Food and Drugs Administration; GC, glucocorticoids; IM, immunomodulatory medication; IVIg, intravenous immunoglobulin; mg, milligrams; MMF, mycophenolate mofetil; N/A, not available; rese-cel, resecabtagene autoleucel; TAC, tacrolimus; TIS, total improvement score. 1. Cabaletta Bio: Data on File. 2. Pinal-Fernandez I, et al. Ann Rheum Dis. 2024;83(11):1549–1560. 3. Galindo-Feria AS, et al. Best Pract Res Clin Rheumatol. 2022;36(2):101767. 4. Müller, F, et al. Nat Med. 2025:31(6):1793–1797. Major TIS Assessment at Week 16 ASyS (baseline autoantibody) ASyS-1 (Jo-1) ASyS-2 (Jo-1) IM-free Low dose or no GC TIS response Major Minimal Complete and transient B cells depletion Antibody trend† ‡ ‡ Meets pivotal primary endpoint Discontinued all immunomodulators§ ASyS-1 ASyS-2 IVIg, TAC IVIg, MMF, TAC ASyS-2 would not meet pivotal entry criteria based on mild muscle weakness on MMT-8. Maximum TIS score possible is 65. Jo-1 antibody increased from Week 20 to Week 24 Mod. Min. None Pivotal primary endpoint (FDA aligned): Moderate TIS off all IM therapy on no steroids or low dose (50% reduction or ≤7.5 mg/day) TIS (N=2) Responses to CD19-CAR T among some ASyS patients may be time-limited by the recurrence or persistence of pathogenic autoantibodies1–3 from CD19-negative long-lived plasma cells despite complete B cell depletion

Lupus: Unmet Need & Clinical Data
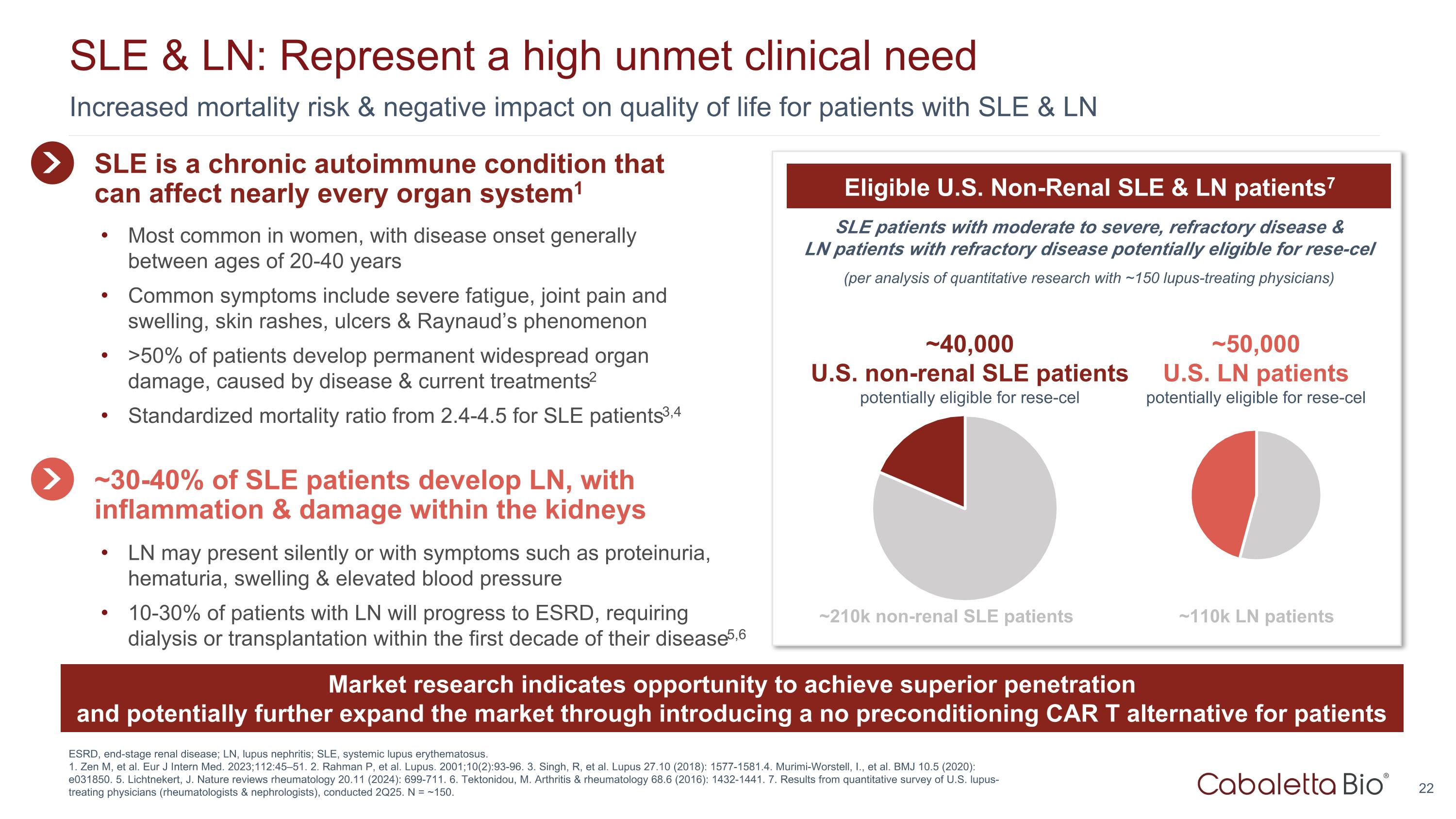
Increased mortality risk & negative impact on quality of life for patients with SLE & LN SLE & LN: Represent a high unmet clinical need ESRD, end-stage renal disease; LN, lupus nephritis; SLE, systemic lupus erythematosus. 1. Zen M, et al. Eur J Intern Med. 2023;112:45–51. 2. Rahman P, et al. Lupus. 2001;10(2):93-96. 3. Singh, R, et al. Lupus 27.10 (2018): 1577-1581.4. Murimi-Worstell, I., et al. BMJ 10.5 (2020): e031850. 5. Lichtnekert, J. Nature reviews rheumatology 20.11 (2024): 699-711. 6. Tektonidou, M. Arthritis & rheumatology 68.6 (2016): 1432-1441. 7. Results from quantitative survey of U.S. lupus-treating physicians (rheumatologists & nephrologists), conducted 2Q25. N = ~150. SLE patients with moderate to severe, refractory disease & LN patients with refractory disease potentially eligible for rese-cel (per analysis of quantitative research with ~150 lupus-treating physicians) Eligible U.S. Non-Renal SLE & LN patients7 ~40,000 U.S. non-renal SLE patients potentially eligible for rese-cel ~210k non-renal SLE patients ~50,000 U.S. LN patients potentially eligible for rese-cel ~110k LN patients SLE is a chronic autoimmune condition that can affect nearly every organ system1 Most common in women, with disease onset generally between ages of 20-40 years Common symptoms include severe fatigue, joint pain and swelling, skin rashes, ulcers & Raynaud’s phenomenon >50% of patients develop permanent widespread organ damage, caused by disease & current treatments2 Standardized mortality ratio from 2.4-4.5 for SLE patients3,4 ~30-40% of SLE patients develop LN, with inflammation & damage within the kidneys LN may present silently or with symptoms such as proteinuria, hematuria, swelling & elevated blood pressure 10-30% of patients with LN will progress to ESRD, requiring dialysis or transplantation within the first decade of their disease5,6 Market research indicates opportunity to achieve superior penetration and potentially further expand the market through introducing a no preconditioning CAR T alternative for patients
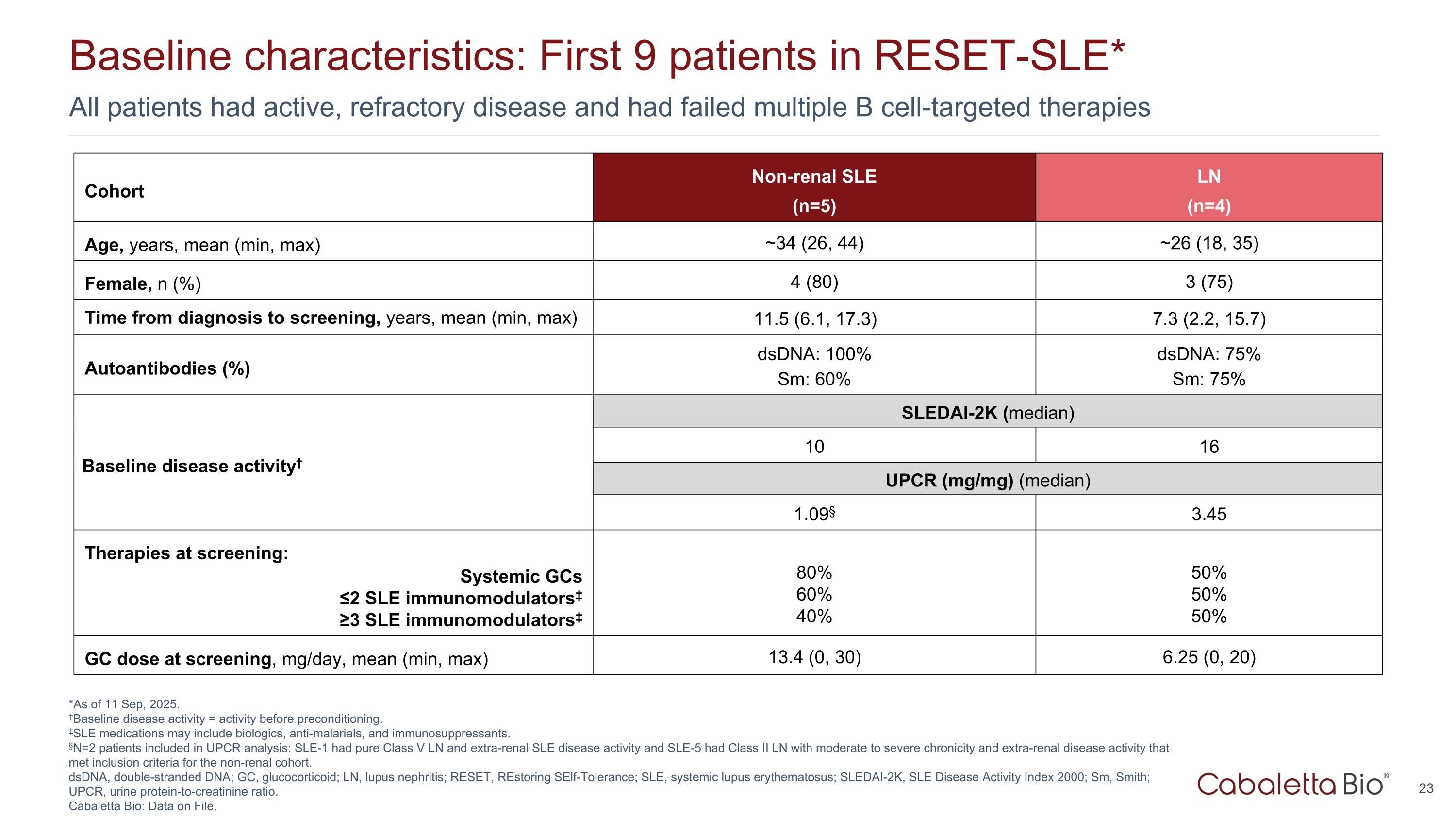
All patients had active, refractory disease and had failed multiple B cell-targeted therapies Baseline characteristics: First 9 patients in RESET-SLE* *As of 11 Sep, 2025. †Baseline disease activity = activity before preconditioning. ‡SLE medications may include biologics, anti-malarials, and immunosuppressants. §N=2 patients included in UPCR analysis: SLE-1 had pure Class V LN and extra-renal SLE disease activity and SLE-5 had Class II LN with moderate to severe chronicity and extra-renal disease activity that met inclusion criteria for the non-renal cohort. dsDNA, double-stranded DNA; GC, glucocorticoid; LN, lupus nephritis; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE Disease Activity Index 2000; Sm, Smith; UPCR, urine protein-to-creatinine ratio. Cabaletta Bio: Data on File. Cohort Non-renal SLE (n=5) LN (n=4) Age, years, mean (min, max) ~34 (26, 44) ~26 (18, 35) Female, n (%) 4 (80) 3 (75) Time from diagnosis to screening, years, mean (min, max) 11.5 (6.1, 17.3) 7.3 (2.2, 15.7) Autoantibodies (%) dsDNA: 100% Sm: 60% dsDNA: 75% Sm: 75% Baseline disease activity† SLEDAI-2K (median) 10 16 UPCR (mg/mg) (median) 1.09§ 3.45 Therapies at screening: Systemic GCs ≤2 SLE immunomodulators‡ ≥3 SLE immunomodulators‡ 80% 60% 40% 50% 50% 50% GC dose at screening, mg/day, mean (min, max) 13.4 (0, 30) 6.25 (0, 20)
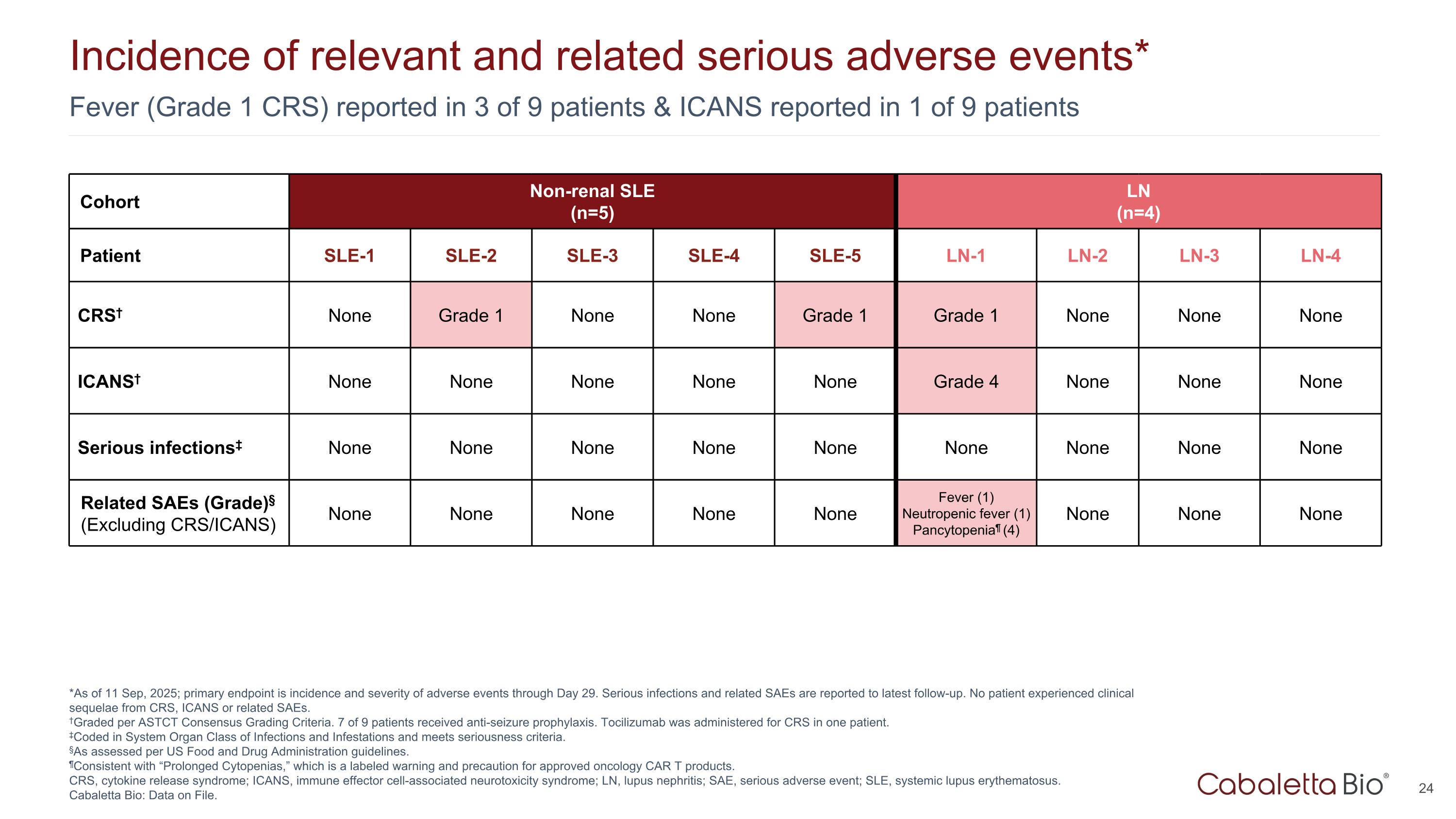
Fever (Grade 1 CRS) reported in 3 of 9 patients & ICANS reported in 1 of 9 patients Incidence of relevant and related serious adverse events* *As of 11 Sep, 2025; primary endpoint is incidence and severity of adverse events through Day 29. Serious infections and related SAEs are reported to latest follow-up. No patient experienced clinical sequelae from CRS, ICANS or related SAEs. †Graded per ASTCT Consensus Grading Criteria. 7 of 9 patients received anti-seizure prophylaxis. Tocilizumab was administered for CRS in one patient. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per US Food and Drug Administration guidelines. ¶Consistent with “Prolonged Cytopenias,” which is a labeled warning and precaution for approved oncology CAR T products. CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; LN, lupus nephritis; SAE, serious adverse event; SLE, systemic lupus erythematosus. Cabaletta Bio: Data on File. Cohort Non-renal SLE (n=5) LN (n=4) Patient SLE-1 SLE-2 SLE-3 SLE-4 SLE-5 LN-1 LN-2 LN-3 LN-4 CRS† None Grade 1 None None Grade 1 Grade 1 None None None ICANS† None None None None None Grade 4 None None None Serious infections‡ None None None None None None None None None Related SAEs (Grade)§ (Excluding CRS/ICANS) None None None None None Fever (1) Neutropenic fever (1) Pancytopenia¶ (4) None None None
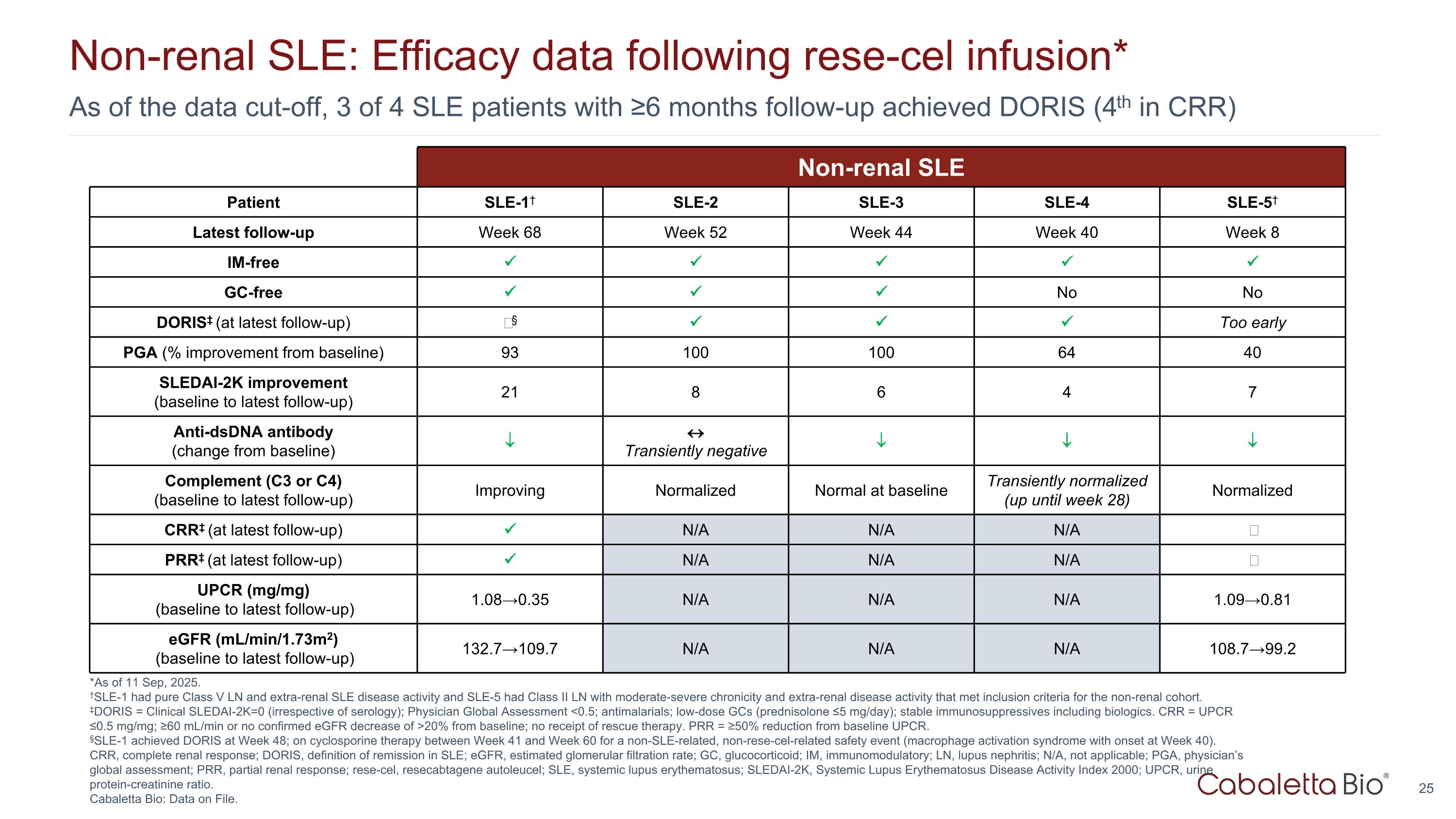
As of the data cut-off, 3 of 4 SLE patients with ≥6 months follow-up achieved DORIS (4th in CRR) Non-renal SLE: Efficacy data following rese-cel infusion* *As of 11 Sep, 2025. †SLE-1 had pure Class V LN and extra-renal SLE disease activity and SLE-5 had Class II LN with moderate-severe chronicity and extra-renal disease activity that met inclusion criteria for the non-renal cohort. ‡DORIS = Clinical SLEDAI-2K=0 (irrespective of serology); Physician Global Assessment <0.5; antimalarials; low-dose GCs (prednisolone ≤5 mg/day); stable immunosuppressives including biologics. CRR = UPCR ≤0.5 mg/mg; ≥60 mL/min or no confirmed eGFR decrease of >20% from baseline; no receipt of rescue therapy. PRR = ≥50% reduction from baseline UPCR. §SLE-1 achieved DORIS at Week 48; on cyclosporine therapy between Week 41 and Week 60 for a non-SLE-related, non-rese-cel-related safety event (macrophage activation syndrome with onset at Week 40). CRR, complete renal response; DORIS, definition of remission in SLE; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; IM, immunomodulatory; LN, lupus nephritis; N/A, not applicable; PGA, physician’s global assessment; PRR, partial renal response; rese-cel, resecabtagene autoleucel; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urine protein-creatinine ratio. Cabaletta Bio: Data on File. Non-renal SLE Patient SLE-1† SLE-2 SLE-3 SLE-4 SLE-5† Latest follow-up Week 68 Week 52 Week 44 Week 40 Week 8 IM-free GC-free No No DORIS‡ (at latest follow-up) ‒§ Too early PGA (% improvement from baseline) 93 100 100 64 40 SLEDAI-2K improvement (baseline to latest follow-up) 21 8 6 4 7 Anti-dsDNA antibody (change from baseline) Transiently negative Complement (C3 or C4) (baseline to latest follow-up) Improving Normalized Normal at baseline Transiently normalized (up until week 28) Normalized CRR‡ (at latest follow-up) N/A N/A N/A ‒ PRR‡ (at latest follow-up) N/A N/A N/A ‒ UPCR (mg/mg) (baseline to latest follow-up) 1.08→0.35 N/A N/A N/A 1.09→0.81 eGFR (mL/min/1.73m2) (baseline to latest follow-up) 132.7→109.7 N/A N/A N/A 108.7→99.2
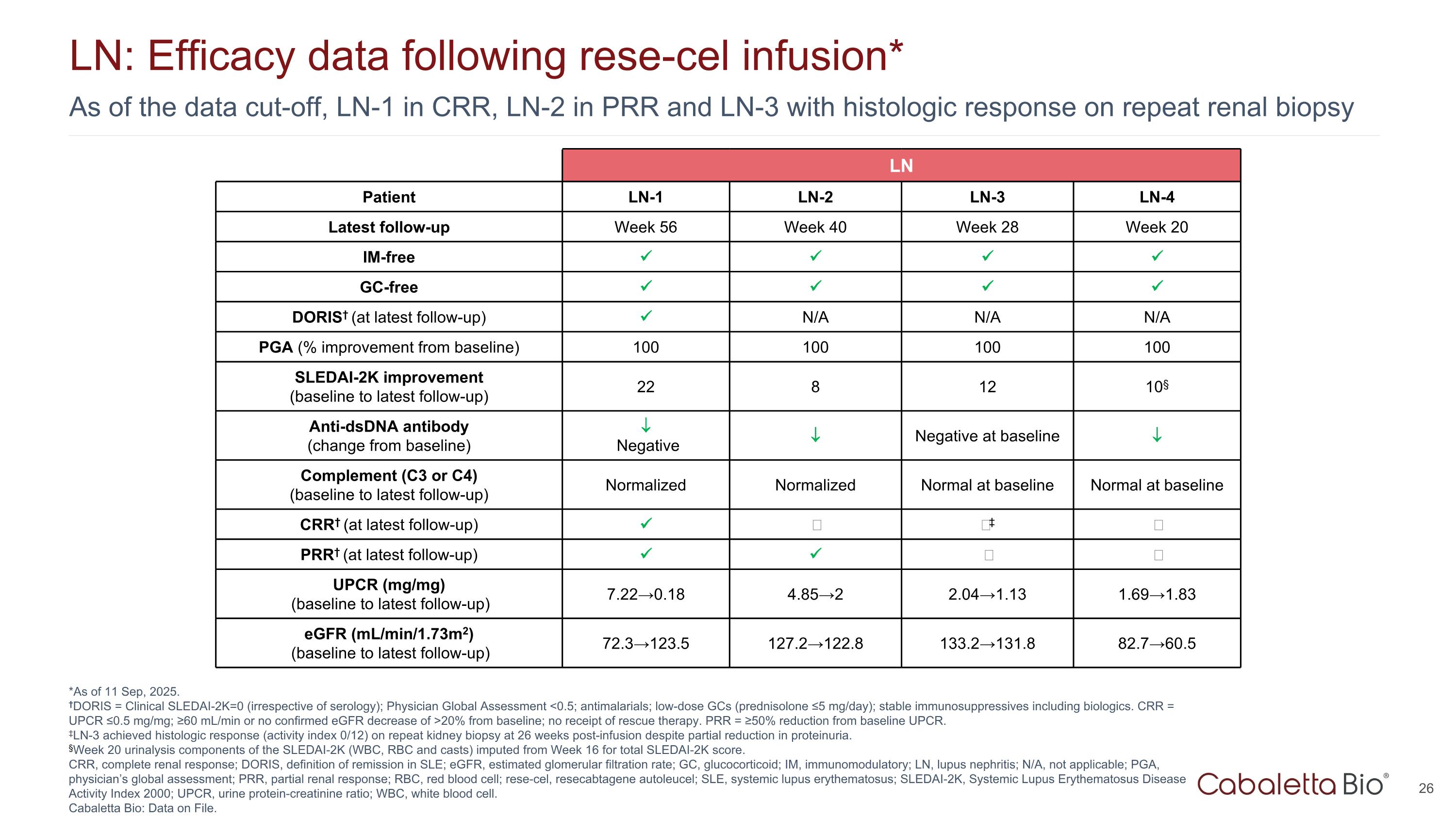
As of the data cut-off, LN-1 in CRR, LN-2 in PRR and LN-3 with histologic response on repeat renal biopsy LN: Efficacy data following rese-cel infusion* *As of 11 Sep, 2025. †DORIS = Clinical SLEDAI-2K=0 (irrespective of serology); Physician Global Assessment <0.5; antimalarials; low-dose GCs (prednisolone ≤5 mg/day); stable immunosuppressives including biologics. CRR = UPCR ≤0.5 mg/mg; ≥60 mL/min or no confirmed eGFR decrease of >20% from baseline; no receipt of rescue therapy. PRR = ≥50% reduction from baseline UPCR. ‡LN-3 achieved histologic response (activity index 0/12) on repeat kidney biopsy at 26 weeks post-infusion despite partial reduction in proteinuria. §Week 20 urinalysis components of the SLEDAI-2K (WBC, RBC and casts) imputed from Week 16 for total SLEDAI-2K score. CRR, complete renal response; DORIS, definition of remission in SLE; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; IM, immunomodulatory; LN, lupus nephritis; N/A, not applicable; PGA, physician’s global assessment; PRR, partial renal response; RBC, red blood cell; rese-cel, resecabtagene autoleucel; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urine protein-creatinine ratio; WBC, white blood cell. Cabaletta Bio: Data on File. LN Patient LN-1 LN-2 LN-3 LN-4 Latest follow-up Week 56 Week 40 Week 28 Week 20 IM-free GC-free DORIS† (at latest follow-up) N/A N/A N/A PGA (% improvement from baseline) 100 100 100 100 SLEDAI-2K improvement (baseline to latest follow-up) 22 8 12 10§ Anti-dsDNA antibody (change from baseline) Negative Negative at baseline Complement (C3 or C4) (baseline to latest follow-up) Normalized Normalized Normal at baseline Normal at baseline CRR† (at latest follow-up) ‒ ‒‡ ‒ PRR† (at latest follow-up) ‒ ‒ UPCR (mg/mg) (baseline to latest follow-up) 7.22→0.18 4.85→2 2.04→1.13 1.69→1.83 eGFR (mL/min/1.73m2) (baseline to latest follow-up) 72.3→123.5 127.2→122.8 133.2→131.8 82.7→60.5
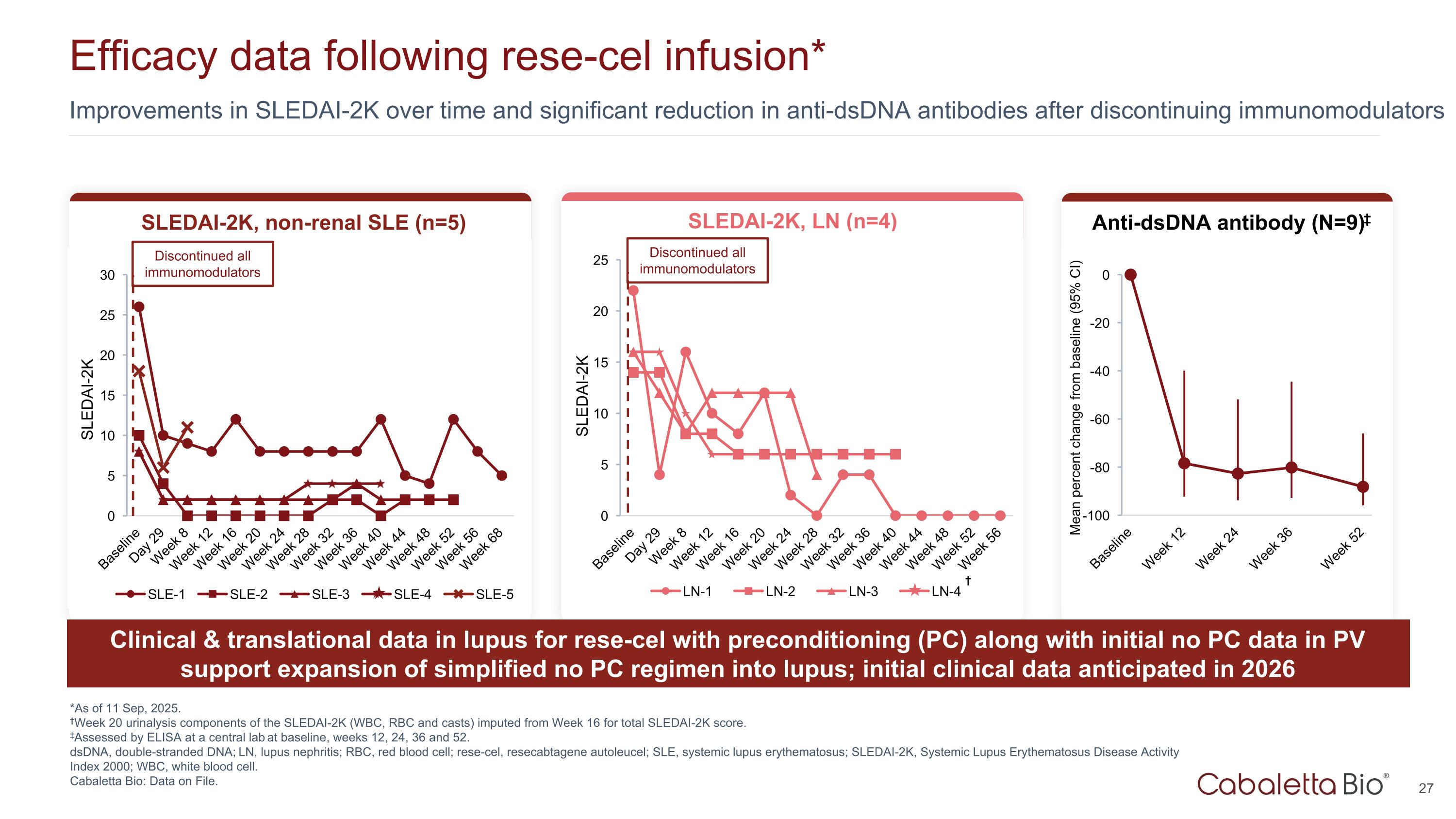
Improvements in SLEDAI-2K over time and significant reduction in anti-dsDNA antibodies after discontinuing immunomodulators Efficacy data following rese-cel infusion* *As of 11 Sep, 2025. †Week 20 urinalysis components of the SLEDAI-2K (WBC, RBC and casts) imputed from Week 16 for total SLEDAI-2K score. ‡Assessed by ELISA at a central lab at baseline, weeks 12, 24, 36 and 52. dsDNA, double-stranded DNA; LN, lupus nephritis; RBC, red blood cell; rese-cel, resecabtagene autoleucel; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; WBC, white blood cell. Cabaletta Bio: Data on File. SLEDAI-2K, non-renal SLE (n=5) SLEDAI-2K Discontinued all immunomodulators SLEDAI-2K, LN (n=4) Anti-dsDNA antibody (N=9)‡ SLEDAI-2K Discontinued all immunomodulators † Clinical & translational data in lupus for rese-cel with preconditioning (PC) along with initial no PC data in PV support expansion of simplified no PC regimen into lupus; initial clinical data anticipated in 2026

Systemic Sclerosis: Unmet Need & Clinical Data
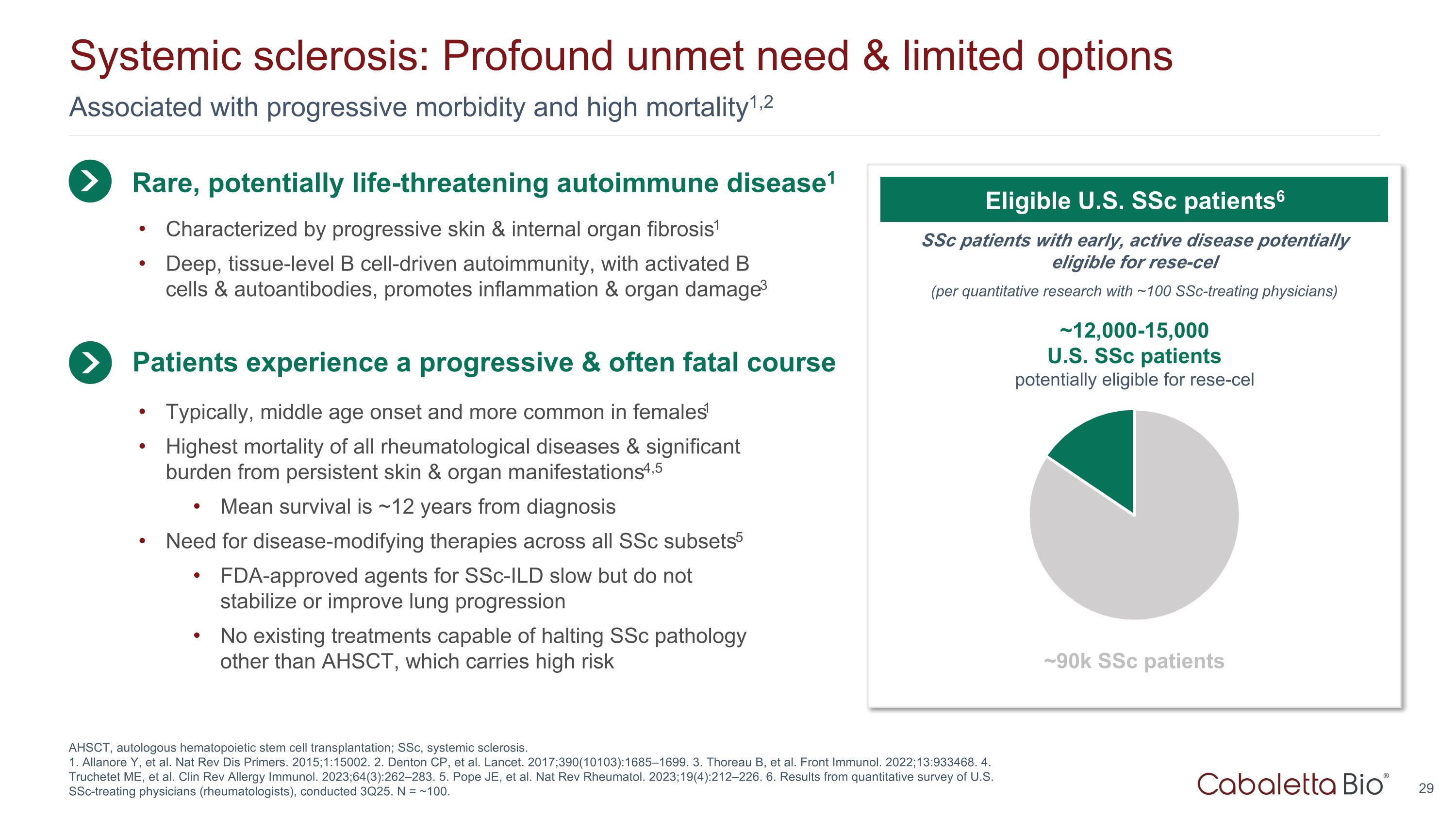
Associated with progressive morbidity and high mortality1,2 Systemic sclerosis: Profound unmet need & limited options AHSCT, autologous hematopoietic stem cell transplantation; SSc, systemic sclerosis. 1. Allanore Y, et al. Nat Rev Dis Primers. 2015;1:15002. 2. Denton CP, et al. Lancet. 2017;390(10103):1685–1699. 3. Thoreau B, et al. Front Immunol. 2022;13:933468. 4. Truchetet ME, et al. Clin Rev Allergy Immunol. 2023;64(3):262–283. 5. Pope JE, et al. Nat Rev Rheumatol. 2023;19(4):212–226. 6. Results from quantitative survey of U.S. SSc-treating physicians (rheumatologists), conducted 3Q25. N = ~100. Rare, potentially life-threatening autoimmune disease1 Typically, middle age onset and more common in females1 Highest mortality of all rheumatological diseases & significant burden from persistent skin & organ manifestations4,5 Mean survival is ~12 years from diagnosis Need for disease-modifying therapies across all SSc subsets5 FDA-approved agents for SSc-ILD slow but do not stabilize or improve lung progression No existing treatments capable of halting SSc pathology other than AHSCT, which carries high risk Patients experience a progressive & often fatal course SSc patients with early, active disease potentially eligible for rese-cel (per quantitative research with ~100 SSc-treating physicians) Eligible U.S. SSc patients6 ~12,000-15,000 U.S. SSc patients potentially eligible for rese-cel ~90k SSc patients Characterized by progressive skin & internal organ fibrosis1 Deep, tissue-level B cell-driven autoimmunity, with activated B cells & autoantibodies, promotes inflammation & organ damage3
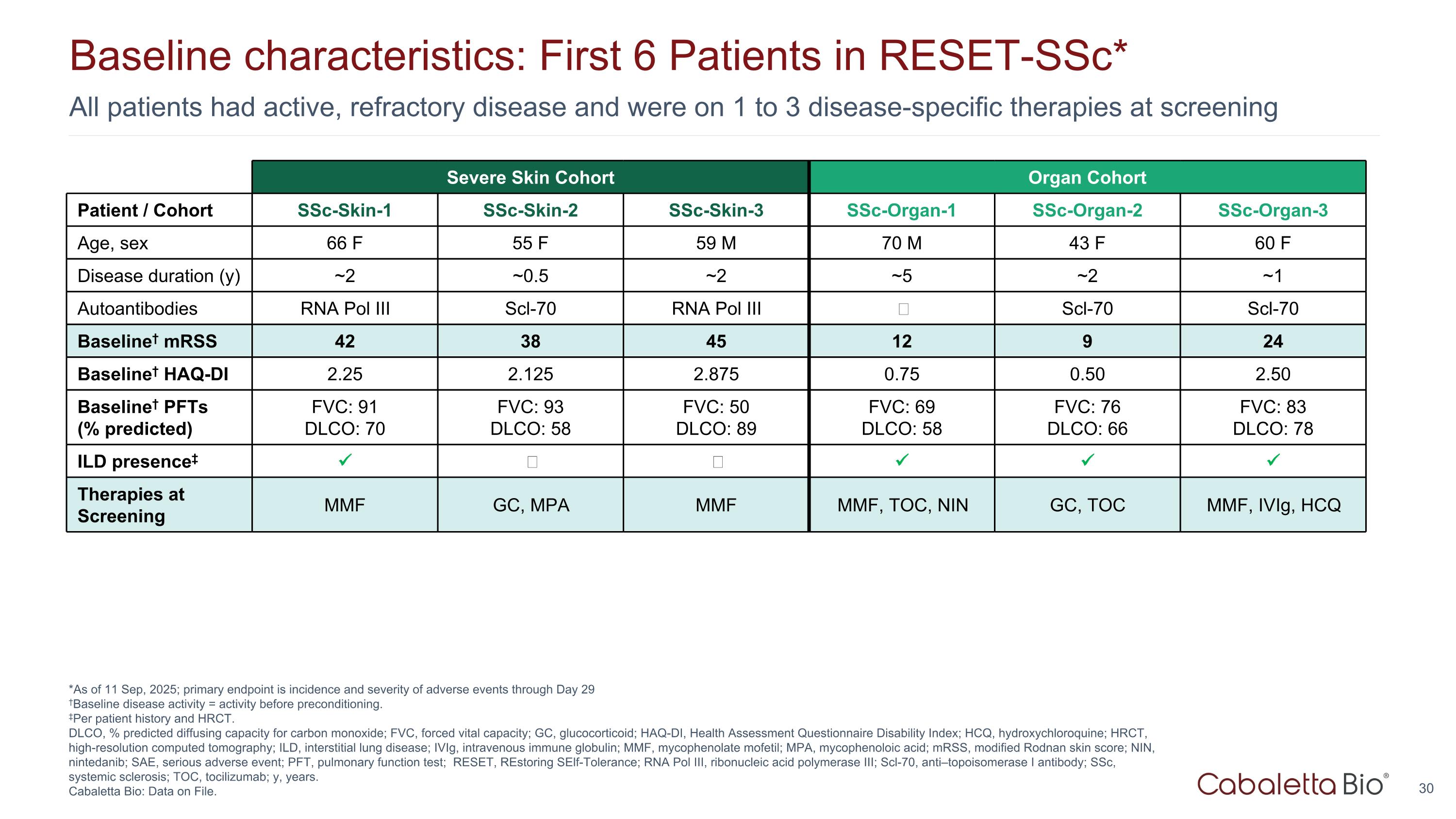
All patients had active, refractory disease and were on 1 to 3 disease-specific therapies at screening Baseline characteristics: First 6 Patients in RESET-SSc* *As of 11 Sep, 2025; primary endpoint is incidence and severity of adverse events through Day 29 †Baseline disease activity = activity before preconditioning. ‡Per patient history and HRCT. DLCO, % predicted diffusing capacity for carbon monoxide; FVC, forced vital capacity; GC, glucocorticoid; HAQ-DI, Health Assessment Questionnaire Disability Index; HCQ, hydroxychloroquine; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; IVIg, intravenous immune globulin; MMF, mycophenolate mofetil; MPA, mycophenoloic acid; mRSS, modified Rodnan skin score; NIN, nintedanib; SAE, serious adverse event; PFT, pulmonary function test; RESET, REstoring SElf-Tolerance; RNA Pol III, ribonucleic acid polymerase III; Scl-70, anti–topoisomerase I antibody; SSc, systemic sclerosis; TOC, tocilizumab; y, years. Cabaletta Bio: Data on File. Severe Skin Cohort Organ Cohort Patient / Cohort SSc-Skin-1 SSc-Skin-2 SSc-Skin-3 SSc-Organ-1 SSc-Organ-2 SSc-Organ-3 Age, sex 66 F 55 F 59 M 70 M 43 F 60 F Disease duration (y) ~2 ~0.5 ~2 ~5 ~2 ~1 Autoantibodies RNA Pol III Scl-70 RNA Pol III ‒ Scl-70 Scl-70 Baseline† mRSS 42 38 45 12 9 24 Baseline† HAQ-DI 2.25 2.125 2.875 0.75 0.50 2.50 Baseline† PFTs (% predicted) FVC: 91 DLCO: 70 FVC: 93 DLCO: 58 FVC: 50 DLCO: 89 FVC: 69 DLCO: 58 FVC: 76 DLCO: 66 FVC: 83 DLCO: 78 ILD presence‡ ‒ ‒ Therapies at Screening MMF GC, MPA MMF MMF, TOC, NIN GC, TOC MMF, IVIg, HCQ
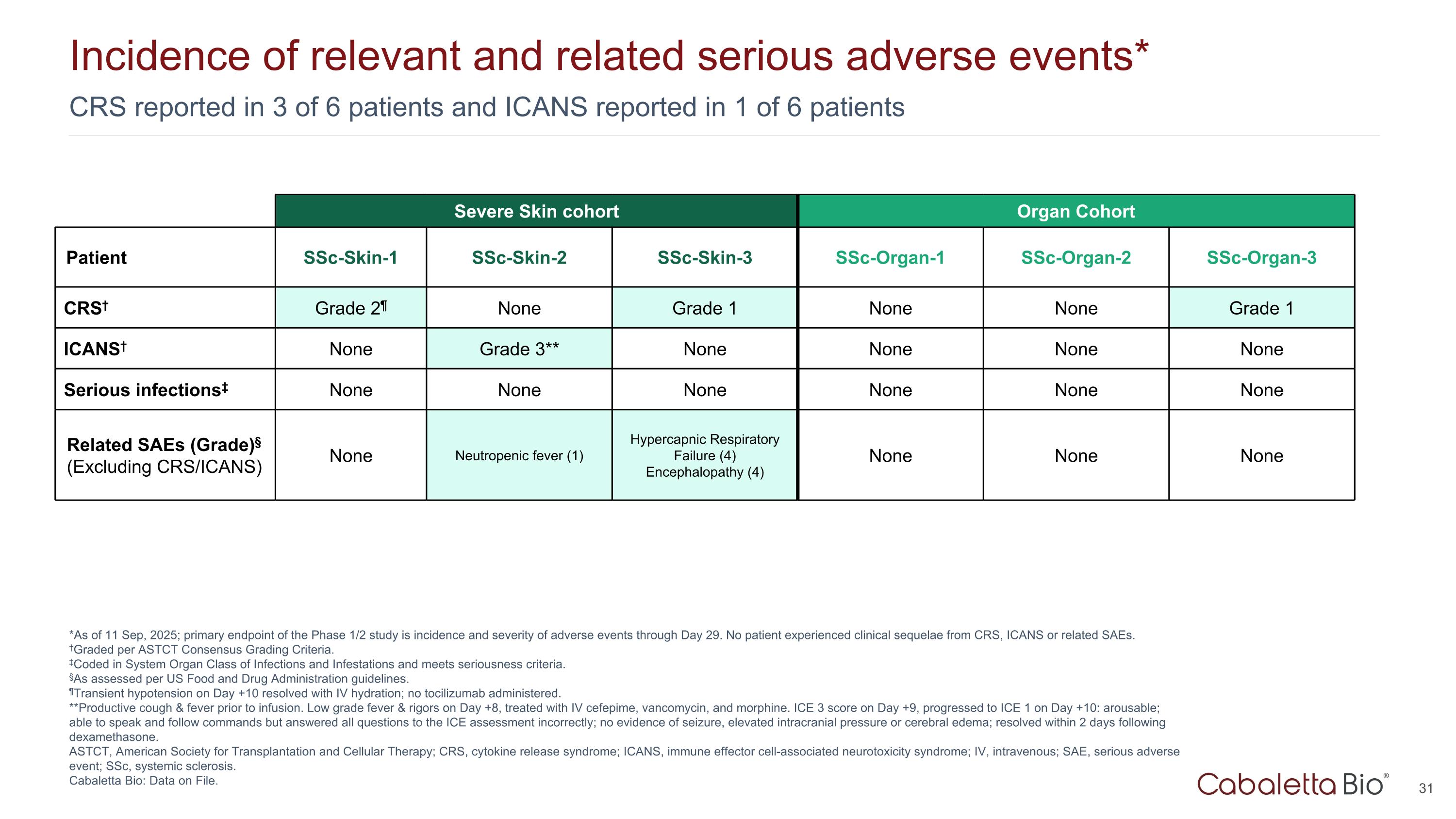
CRS reported in 3 of 6 patients and ICANS reported in 1 of 6 patients Incidence of relevant and related serious adverse events* *As of 11 Sep, 2025; primary endpoint of the Phase 1/2 study is incidence and severity of adverse events through Day 29. No patient experienced clinical sequelae from CRS, ICANS or related SAEs. †Graded per ASTCT Consensus Grading Criteria. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per US Food and Drug Administration guidelines. ¶Transient hypotension on Day +10 resolved with IV hydration; no tocilizumab administered. **Productive cough & fever prior to infusion. Low grade fever & rigors on Day +8, treated with IV cefepime, vancomycin, and morphine. ICE 3 score on Day +9, progressed to ICE 1 on Day +10: arousable; able to speak and follow commands but answered all questions to the ICE assessment incorrectly; no evidence of seizure, elevated intracranial pressure or cerebral edema; resolved within 2 days following dexamethasone. ASTCT, American Society for Transplantation and Cellular Therapy; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; IV, intravenous; SAE, serious adverse event; SSc, systemic sclerosis. Cabaletta Bio: Data on File. Severe Skin cohort Organ Cohort Patient SSc-Skin-1 SSc-Skin-2 SSc-Skin-3 SSc-Organ-1 SSc-Organ-2 SSc-Organ-3 CRS† Grade 2¶ None Grade 1 None None Grade 1 ICANS† None Grade 3** None None None None Serious infections‡ None None None None None None Related SAEs (Grade)§ (Excluding CRS/ICANS) None Neutropenic fever (1) Hypercapnic Respiratory Failure (4) Encephalopathy (4) None None None
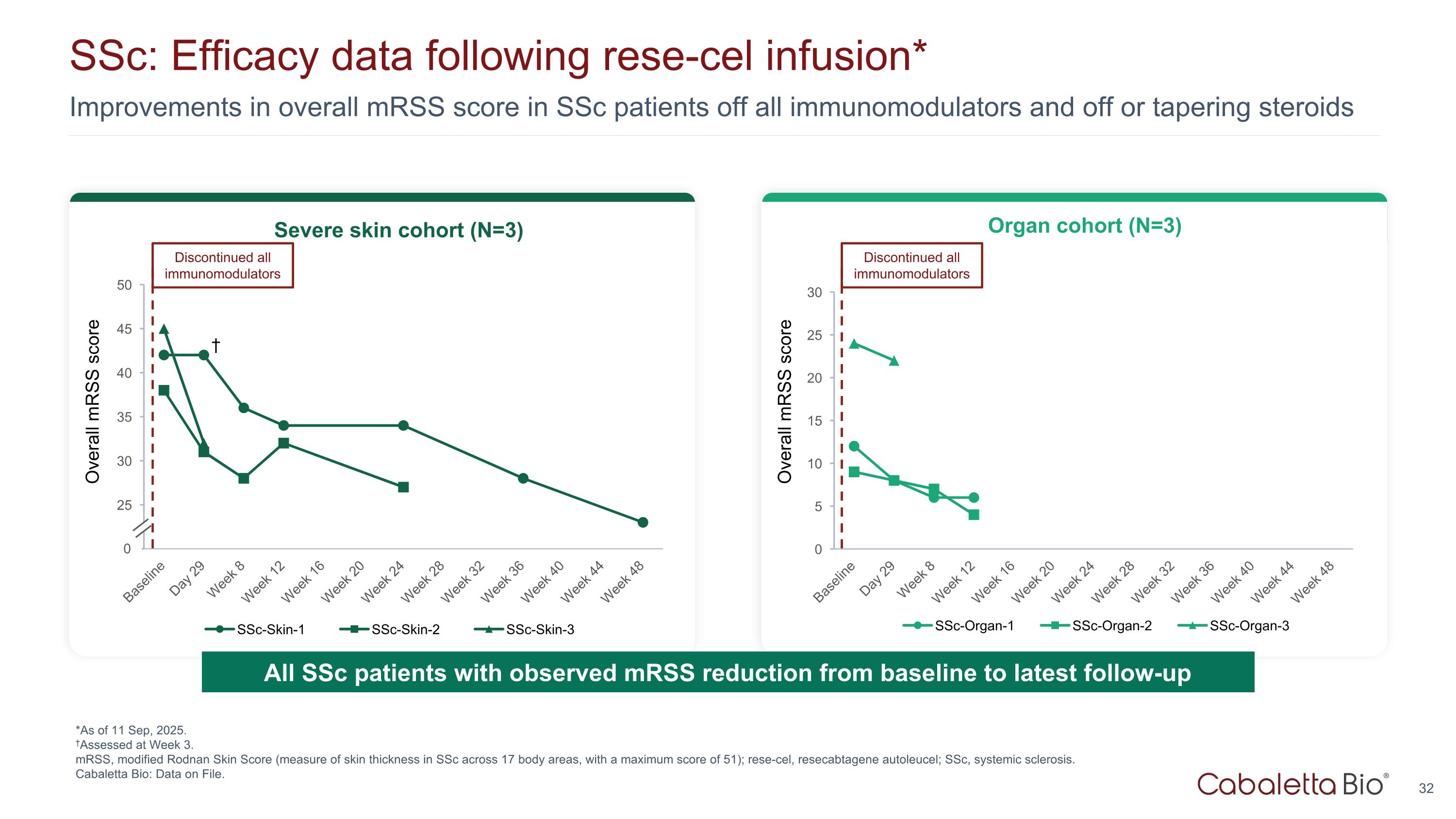
Improvements in overall mRSS score in SSc patients off all immunomodulators and off or tapering steroids SSc: Efficacy data following rese-cel infusion* *As of 11 Sep, 2025. †Assessed at Week 3. mRSS, modified Rodnan Skin Score (measure of skin thickness in SSc across 17 body areas, with a maximum score of 51); rese-cel, resecabtagene autoleucel; SSc, systemic sclerosis. Cabaletta Bio: Data on File. Overall mRSS score Severe skin cohort (N=3) Organ cohort (N=3) 0 Discontinued all immunomodulators † Overall mRSS score Discontinued all immunomodulators All SSc patients with observed mRSS reduction from baseline to latest follow-up
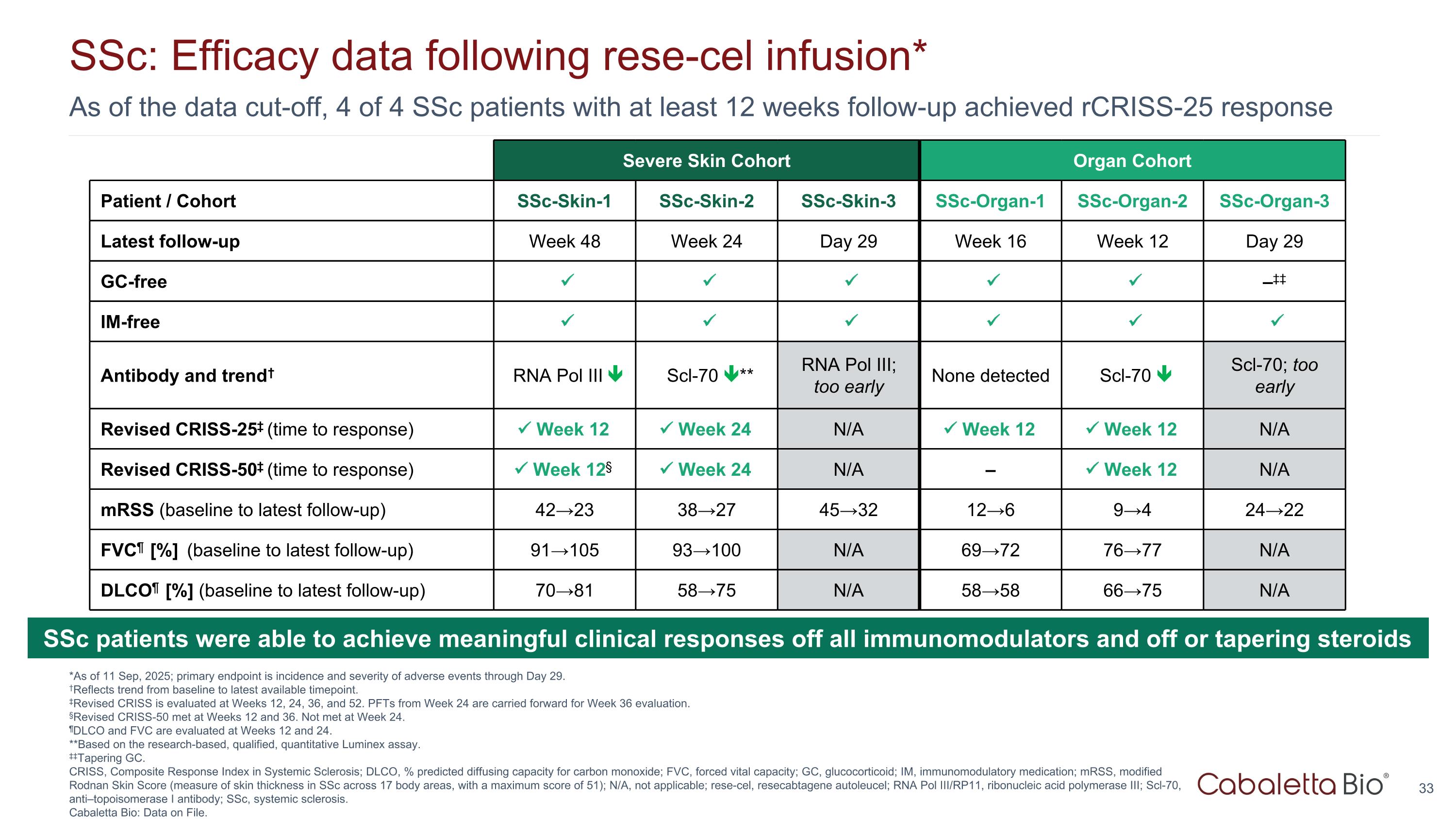
As of the data cut-off, 4 of 4 SSc patients with at least 12 weeks follow-up achieved rCRISS-25 response SSc: Efficacy data following rese-cel infusion* *As of 11 Sep, 2025; primary endpoint is incidence and severity of adverse events through Day 29. †Reflects trend from baseline to latest available timepoint. ‡Revised CRISS is evaluated at Weeks 12, 24, 36, and 52. PFTs from Week 24 are carried forward for Week 36 evaluation. §Revised CRISS-50 met at Weeks 12 and 36. Not met at Week 24. ¶DLCO and FVC are evaluated at Weeks 12 and 24. **Based on the research-based, qualified, quantitative Luminex assay. ‡‡Tapering GC. CRISS, Composite Response Index in Systemic Sclerosis; DLCO, % predicted diffusing capacity for carbon monoxide; FVC, forced vital capacity; GC, glucocorticoid; IM, immunomodulatory medication; mRSS, modified Rodnan Skin Score (measure of skin thickness in SSc across 17 body areas, with a maximum score of 51); N/A, not applicable; rese-cel, resecabtagene autoleucel; RNA Pol III/RP11, ribonucleic acid polymerase III; Scl-70, anti–topoisomerase I antibody; SSc, systemic sclerosis. Cabaletta Bio: Data on File. Severe Skin Cohort Organ Cohort Patient / Cohort SSc-Skin-1 SSc-Skin-2 SSc-Skin-3 SSc-Organ-1 SSc-Organ-2 SSc-Organ-3 Latest follow-up Week 48 Week 24 Day 29 Week 16 Week 12 Day 29 GC-free –‡‡ IM-free Antibody and trend† RNA Pol III Scl-70 ** RNA Pol III; too early None detected Scl-70 Scl-70; too early Revised CRISS-25‡ (time to response) Week 12 Week 24 N/A Week 12 Week 12 N/A Revised CRISS-50‡ (time to response) Week 12§ Week 24 N/A – Week 12 N/A mRSS (baseline to latest follow-up) 42→23 38→27 45→32 12→6 9→4 24→22 FVC¶ [%] (baseline to latest follow-up) 91→105 93→100 N/A 69→72 76→77 N/A DLCO¶ [%] (baseline to latest follow-up) 70→81 58→75 N/A 58→58 66→75 N/A SSc patients were able to achieve meaningful clinical responses off all immunomodulators and off or tapering steroids
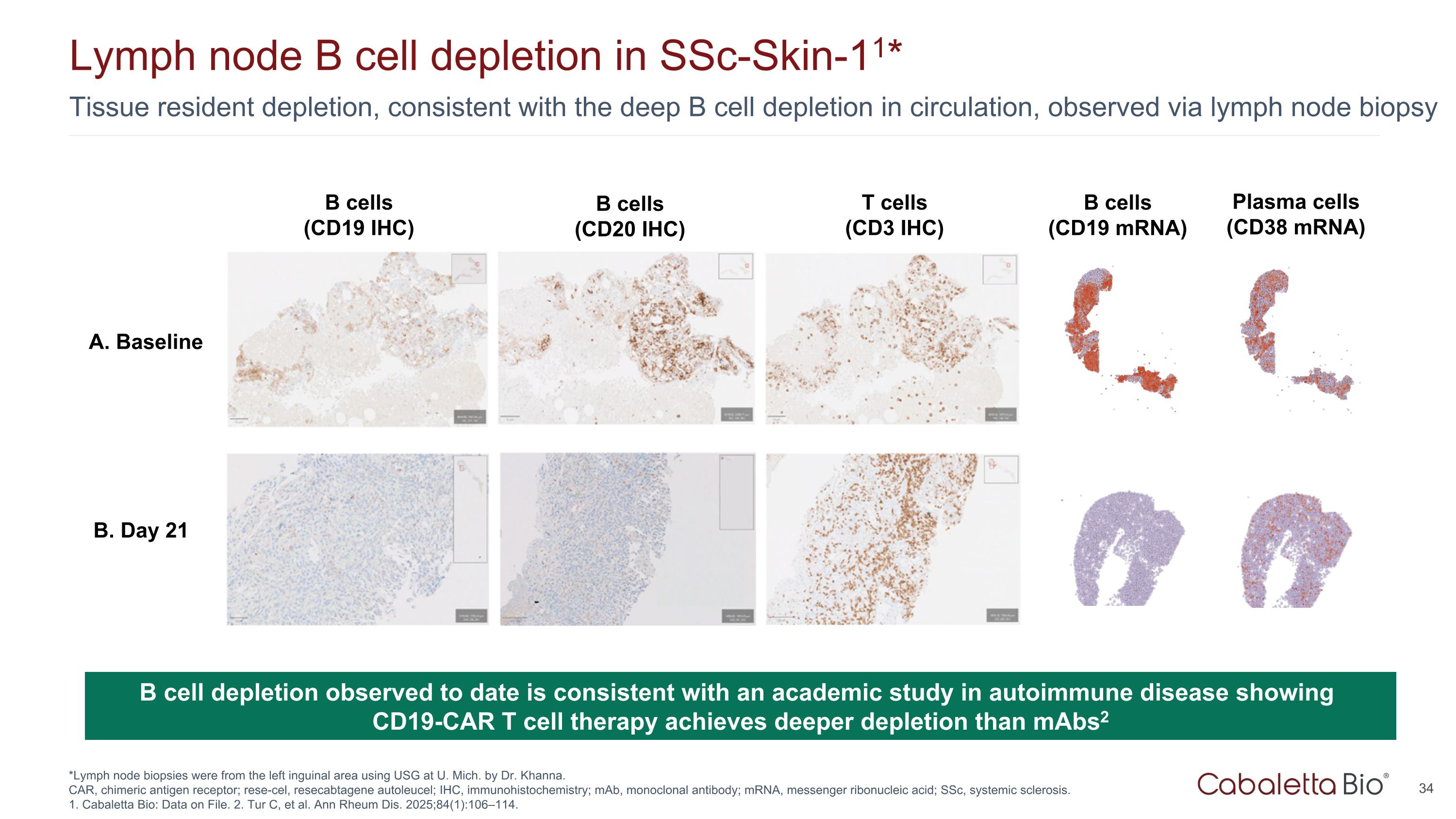
Tissue resident depletion, consistent with the deep B cell depletion in circulation, observed via lymph node biopsy Lymph node B cell depletion in SSc-Skin-11* *Lymph node biopsies were from the left inguinal area using USG at U. Mich. by Dr. Khanna. CAR, chimeric antigen receptor; rese-cel, resecabtagene autoleucel; IHC, immunohistochemistry; mAb, monoclonal antibody; mRNA, messenger ribonucleic acid; SSc, systemic sclerosis. 1. Cabaletta Bio: Data on File. 2. Tur C, et al. Ann Rheum Dis. 2025;84(1):106–114. B cell depletion observed to date is consistent with an academic study in autoimmune disease showing CD19-CAR T cell therapy achieves deeper depletion than mAbs2 A. Baseline B. Day 21 B cells (CD19 mRNA) Plasma cells (CD38 mRNA) B cells (CD19 IHC) B cells (CD20 IHC) T cells (CD3 IHC)

Myasthenia Gravis: Unmet Need
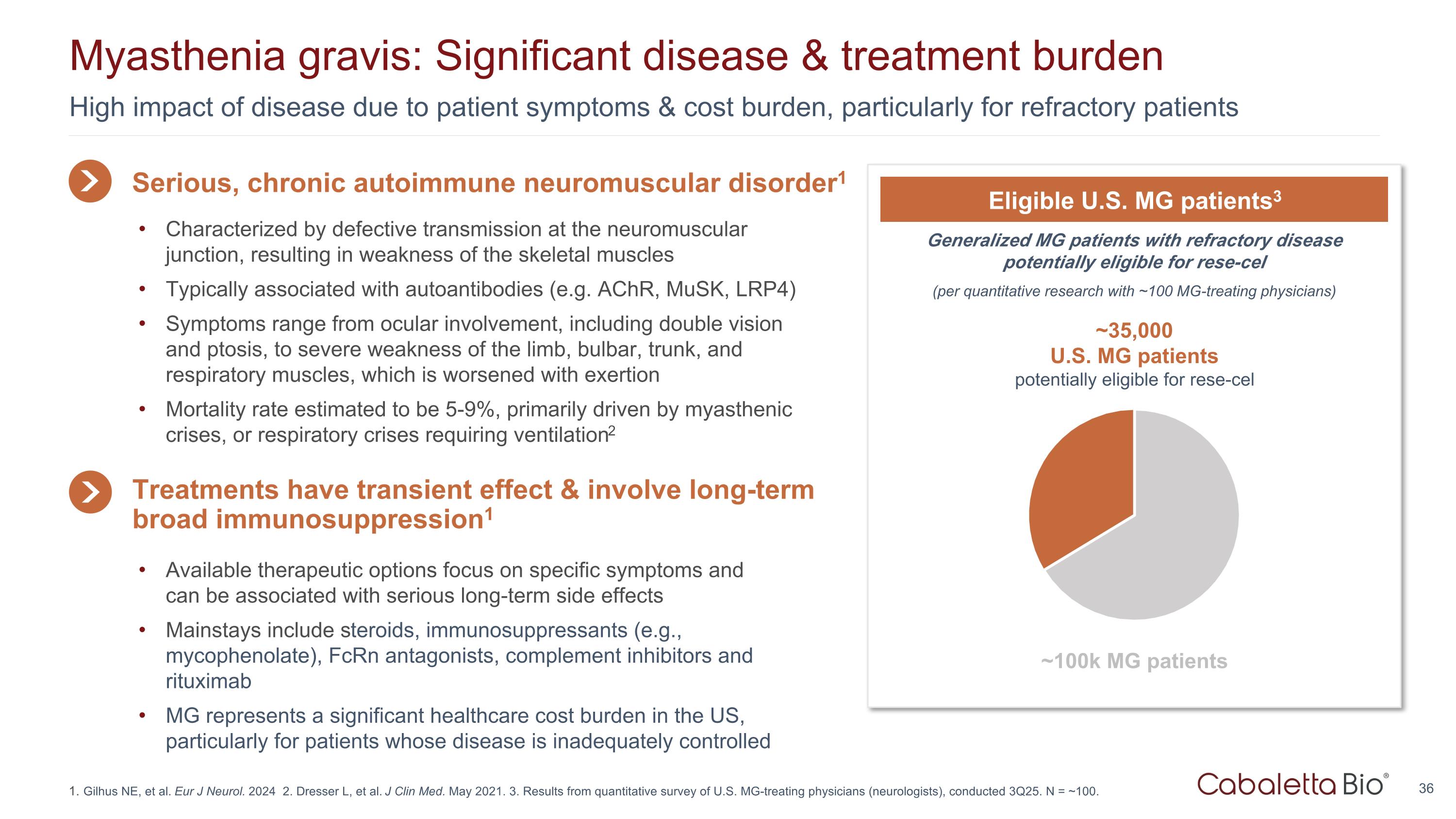
High impact of disease due to patient symptoms & cost burden, particularly for refractory patients Myasthenia gravis: Significant disease & treatment burden Gilhus NE, et al. Eur J Neurol. 2024 2. Dresser L, et al. J Clin Med. May 2021. 3. Results from quantitative survey of U.S. MG-treating physicians (neurologists), conducted 3Q25. N = ~100. Generalized MG patients with refractory disease potentially eligible for rese-cel (per quantitative research with ~100 MG-treating physicians) Eligible U.S. MG patients3 ~35,000 U.S. MG patients potentially eligible for rese-cel ~100k MG patients Serious, chronic autoimmune neuromuscular disorder1 Available therapeutic options focus on specific symptoms and can be associated with serious long-term side effects Mainstays include steroids, immunosuppressants (e.g., mycophenolate), FcRn antagonists, complement inhibitors and rituximab MG represents a significant healthcare cost burden in the US, particularly for patients whose disease is inadequately controlled Treatments have transient effect & involve long-term broad immunosuppression1 Characterized by defective transmission at the neuromuscular junction, resulting in weakness of the skeletal muscles Typically associated with autoantibodies (e.g. AChR, MuSK, LRP4) Symptoms range from ocular involvement, including double vision and ptosis, to severe weakness of the limb, bulbar, trunk, and respiratory muscles, which is worsened with exertion Mortality rate estimated to be 5-9%, primarily driven by myasthenic crises, or respiratory crises requiring ventilation2

Rese-cel – Initial Dose Data without Preconditioning
Clear evidence of biologic and clinical activity in all three PV patients in the initial dose cohort PDAI improvements were present in all three and were compelling in two of the three patients All patients remain off all immunomodulators while GCs are being tapered from low doses Complete B cell depletion was observed in the two patients with the greatest clinical response BAFF induction in these two patients was at the low end of the range of rese-cel with PC Rese-cel persistence without PC was similar to patients who received rese-cel with PC Peak persistence was not impacted by absence of PC and occurred slightly later without PC IFNγ induction in non-PC patients was at the higher end of the range observed in PC patients Higher levels may be attributable to higher B cell burden in PV patients and/or absence of preconditioning Rese-cel was generally well tolerated in PV patients without PC1 Based on limited data in the first three patients without PC, CRS rate was similar in rese-cel patients with PC Summary of early clinical and translational data Rese-cel without preconditioning (PC), initial dose cohort* *As of 11 September 2025. Cabaletta Bio: Data on file. BAFF, B cell activating factor; CRS, cytokine release syndrome; GC: glucocorticoids; PDAI, pemphigus disease area index; PV, pemphigus vulgaris; rese-cel, resacabtagene autoleucel; IFNγ, interferon-gamma Standard preconditioning in RESET trials consists of fludarabine 25 mg/m2 x 3 days and cyclophosphamide 1000 mg/m2 x 1 day.
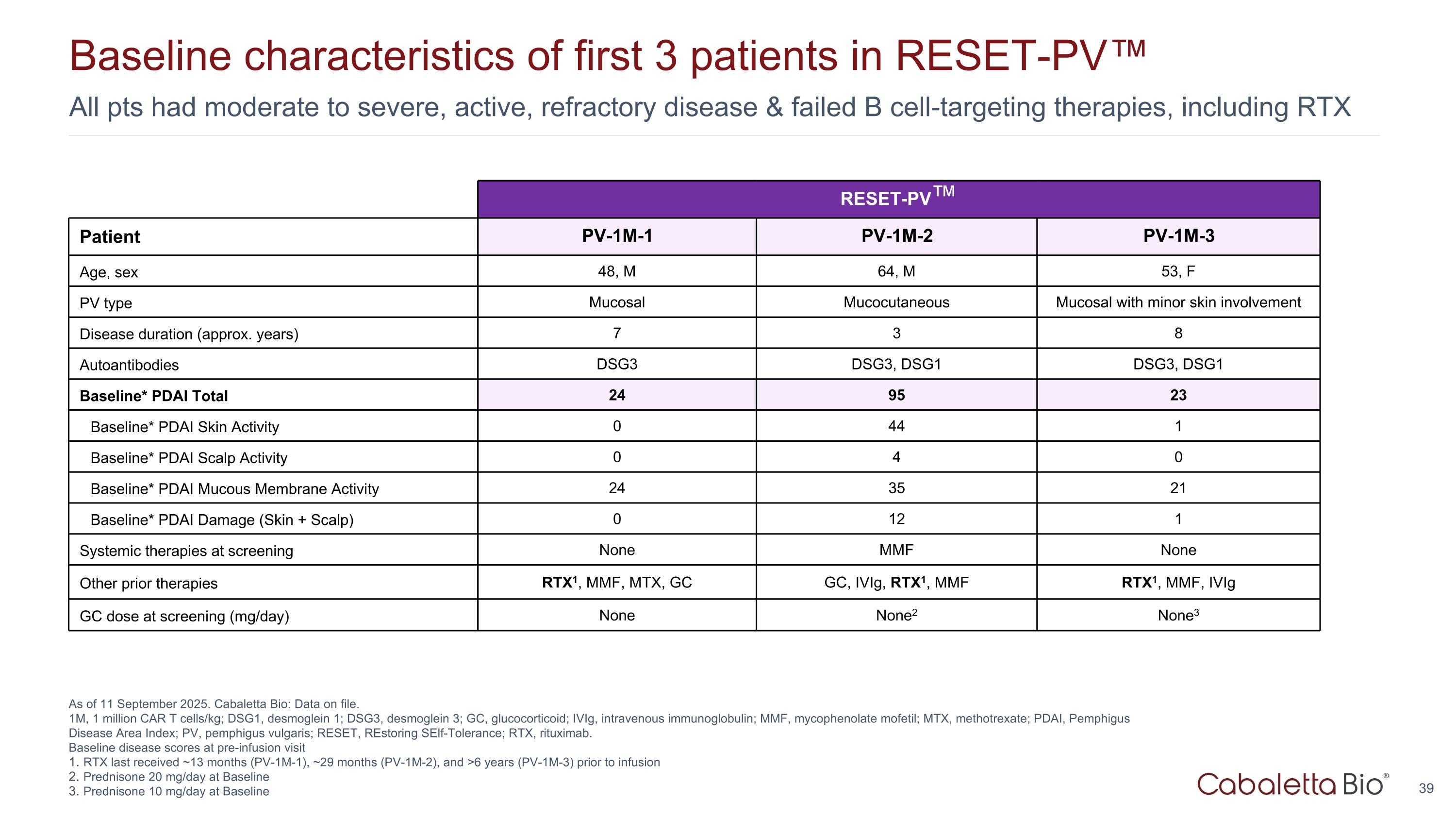
All pts had moderate to severe, active, refractory disease & failed B cell-targeting therapies, including RTX Baseline characteristics of first 3 patients in RESET-PV™ As of 11 September 2025. Cabaletta Bio: Data on file. 1M, 1 million CAR T cells/kg; DSG1, desmoglein 1; DSG3, desmoglein 3; GC, glucocorticoid; IVIg, intravenous immunoglobulin; MMF, mycophenolate mofetil; MTX, methotrexate; PDAI, Pemphigus Disease Area Index; PV, pemphigus vulgaris; RESET, REstoring SElf-Tolerance; RTX, rituximab. Baseline disease scores at pre-infusion visit RTX last received ~13 months (PV-1M-1), ~29 months (PV-1M-2), and >6 years (PV-1M-3) prior to infusion Prednisone 20 mg/day at Baseline Prednisone 10 mg/day at Baseline RESET-PV™ Patient PV-1M-1 PV-1M-2 PV-1M-3 Age, sex 48, M 64, M 53, F PV type Mucosal Mucocutaneous Mucosal with minor skin involvement Disease duration (approx. years) 7 3 8 Autoantibodies DSG3 DSG3, DSG1 DSG3, DSG1 Baseline* PDAI Total 24 95 23 Baseline* PDAI Skin Activity 0 44 1 Baseline* PDAI Scalp Activity 0 4 0 Baseline* PDAI Mucous Membrane Activity 24 35 21 Baseline* PDAI Damage (Skin + Scalp) 0 12 1 Systemic therapies at screening None MMF None Other prior therapies RTX1, MMF, MTX, GC GC, IVIg, RTX1, MMF RTX1, MMF, IVIg GC dose at screening (mg/day) None None2 None3
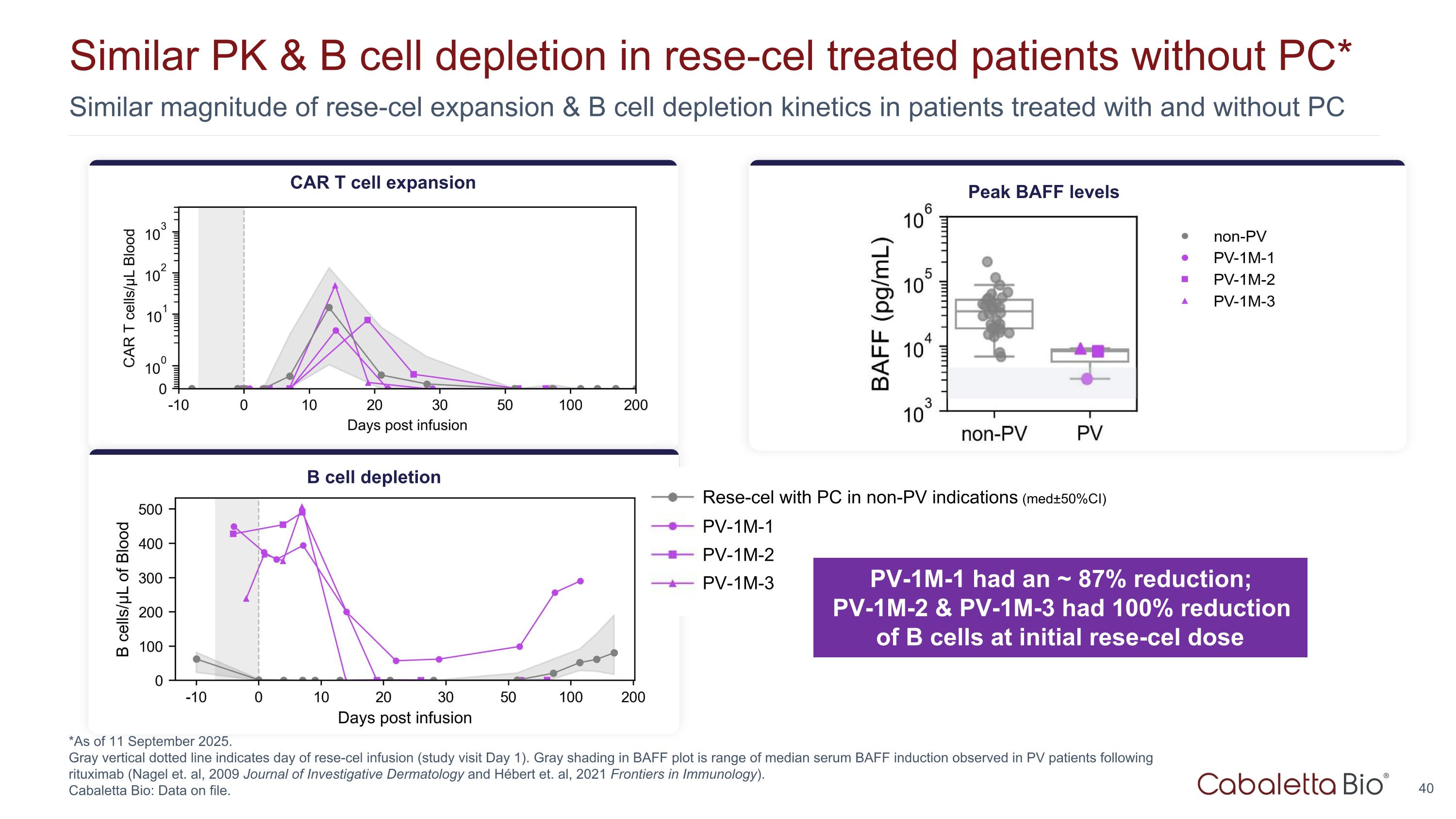
Similar magnitude of rese-cel expansion & B cell depletion kinetics in patients treated with and without PC Similar PK & B cell depletion in rese-cel treated patients without PC* *As of 11 September 2025. Gray vertical dotted line indicates day of rese-cel infusion (study visit Day 1). Gray shading in BAFF plot is range of median serum BAFF induction observed in PV patients following rituximab (Nagel et. al, 2009 Journal of Investigative Dermatology and Hébert et. al, 2021 Frontiers in Immunology). Cabaletta Bio: Data on file. B cell depletion CAR T cell expansion Peak BAFF levels PV-1M-1 had an ~ 87% reduction; PV-1M-2 & PV-1M-3 had 100% reduction of B cells at initial rese-cel dose PV-1M-1 PV-1M-2 PV-1M-3 Rese-cel with PC in non-PV indications (med±50%CI)
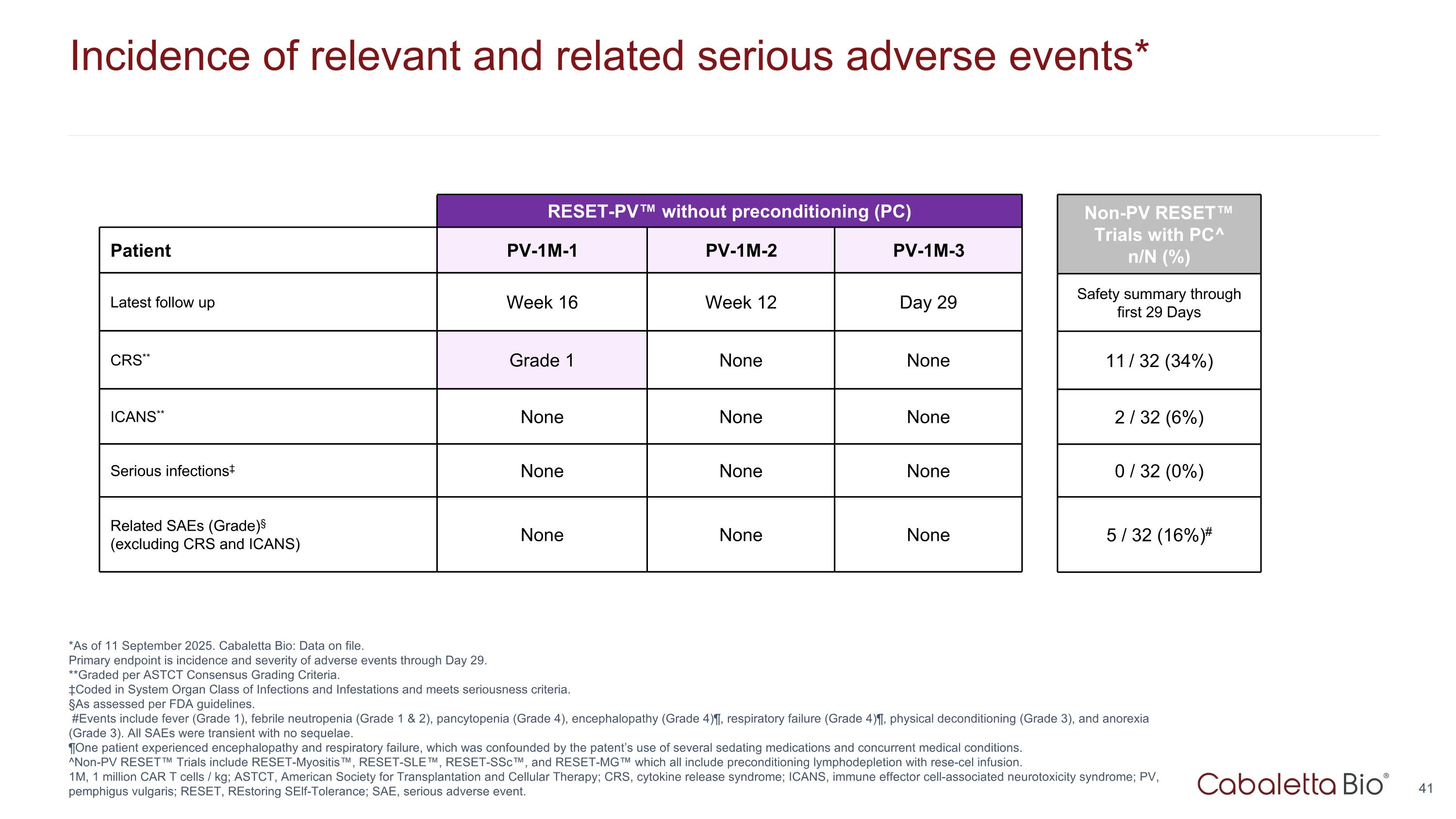
Incidence of relevant and related serious adverse events* *As of 11 September 2025. Cabaletta Bio: Data on file. Primary endpoint is incidence and severity of adverse events through Day 29. **Graded per ASTCT Consensus Grading Criteria. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per FDA guidelines. #Events include fever (Grade 1), febrile neutropenia (Grade 1 & 2), pancytopenia (Grade 4), encephalopathy (Grade 4)¶, respiratory failure (Grade 4)¶, physical deconditioning (Grade 3), and anorexia (Grade 3). All SAEs were transient with no sequelae. ¶One patient experienced encephalopathy and respiratory failure, which was confounded by the patent’s use of several sedating medications and concurrent medical conditions. ^Non-PV RESET™ Trials include RESET-Myositis™, RESET-SLE™, RESET-SSc™, and RESET-MG™ which all include preconditioning lymphodepletion with rese-cel infusion. 1M, 1 million CAR T cells / kg; ASTCT, American Society for Transplantation and Cellular Therapy; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; PV, pemphigus vulgaris; RESET, REstoring SElf-Tolerance; SAE, serious adverse event. RESET-PV™ without preconditioning (PC) RESET-Myositis Patient PV-1M-1 PV-1M-2 PV-1M-3 Latest follow up Week 16 Week 12 Day 29 CRS** Grade 1 None None ICANS** None None None Serious infections‡ None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None Non-PV RESET™ Trials with PC^ n/N (%) Safety summary through first 29 Days 11 / 32 (34%) 2 / 32 (6%) 0 / 32 (0%) 5 / 32 (16%)#
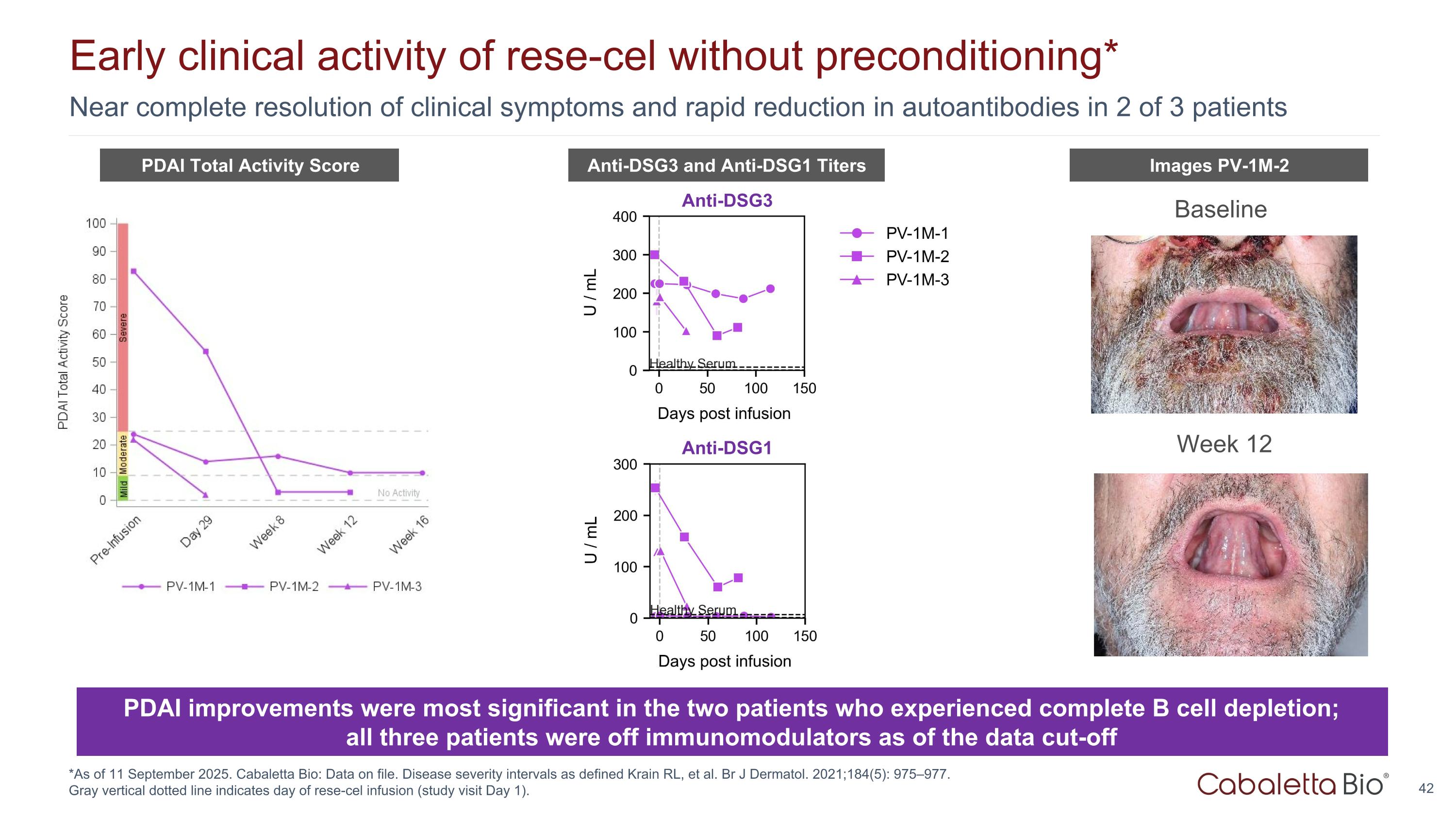
Near complete resolution of clinical symptoms and rapid reduction in autoantibodies in 2 of 3 patients Early clinical activity of rese-cel without preconditioning* *As of 11 September 2025. Cabaletta Bio: Data on file. Disease severity intervals as defined Krain RL, et al. Br J Dermatol. 2021;184(5): 975–977. Gray vertical dotted line indicates day of rese-cel infusion (study visit Day 1). Baseline Week 12 Images PV-1M-2 Images PV-1M-2 PDAI Total Activity Score Anti-DSG3 and Anti-DSG1 Titers PDAI improvements were most significant in the two patients who experienced complete B cell depletion; all three patients were off immunomodulators as of the data cut-off Anti-DSG3 Anti-DSG1

Rese-cel Manufacturing
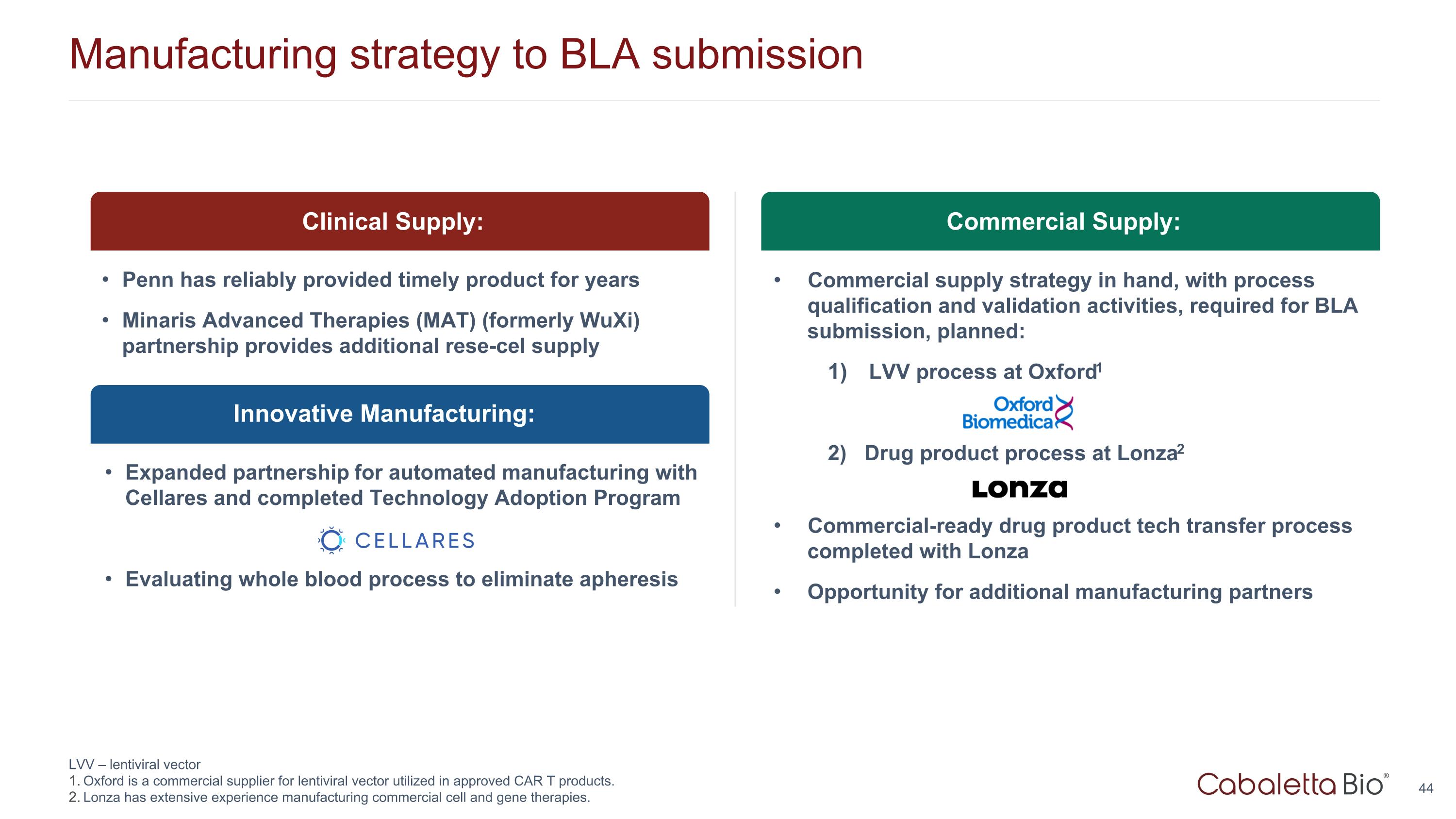
Manufacturing strategy to BLA submission LVV – lentiviral vector Oxford is a commercial supplier for lentiviral vector utilized in approved CAR T products. Lonza has extensive experience manufacturing commercial cell and gene therapies. Innovative Manufacturing: Clinical Supply: Penn has reliably provided timely product for years Minaris Advanced Therapies (MAT) (formerly WuXi) partnership provides additional rese-cel supply Expanded partnership for automated manufacturing with Cellares and completed Technology Adoption Program Evaluating whole blood process to eliminate apheresis Commercial Supply: Commercial supply strategy in hand, with process qualification and validation activities, required for BLA submission, planned: LVV process at Oxford1 2) Drug product process at Lonza2 Commercial-ready drug product tech transfer process completed with Lonza Opportunity for additional manufacturing partners

Corporate Summary

Track record of operational success evaluating & developing novel cell therapy candidates in autoimmunity Cabaletta Bio leadership LEADERSHIP TEAM Anup Marda Chief Financial Officer Nicolette Sherman Chief HR Officer Michael Gerard General Counsel Steven Nichtberger, M.D. President, CEO & Chairman Heather Harte-Hall Chief Compliance Officer Gwendolyn Binder, Ph.D. President, Science & Technology SCIENTIFIC ADVISORY BOARD Aimee Payne, M.D., Ph.D. Co-Founder and Co-Chair Michael C. Milone, M.D., Ph.D. Co-Founder and Co-Chair Carl June, M.D. Jay Siegel, M.D. Drew Weissman, M.D., Ph.D. Iain McInnes, Ph.D., FRCP, FRSE, FMedSci Georg Schett, M.D. David J. Chang, M.D., M.P.H., FACR Chief Medical Officer Samik Basu, M.D. Chief Scientific Officer Arun Das, M.D. Chief Business Officer From Fortune. ©2024 Fortune Media IP Limited. All rights reserved. Used under license. Sarah Yuan Chief Technology Officer Steve Gavel Chief Commercial Officer

Appendix
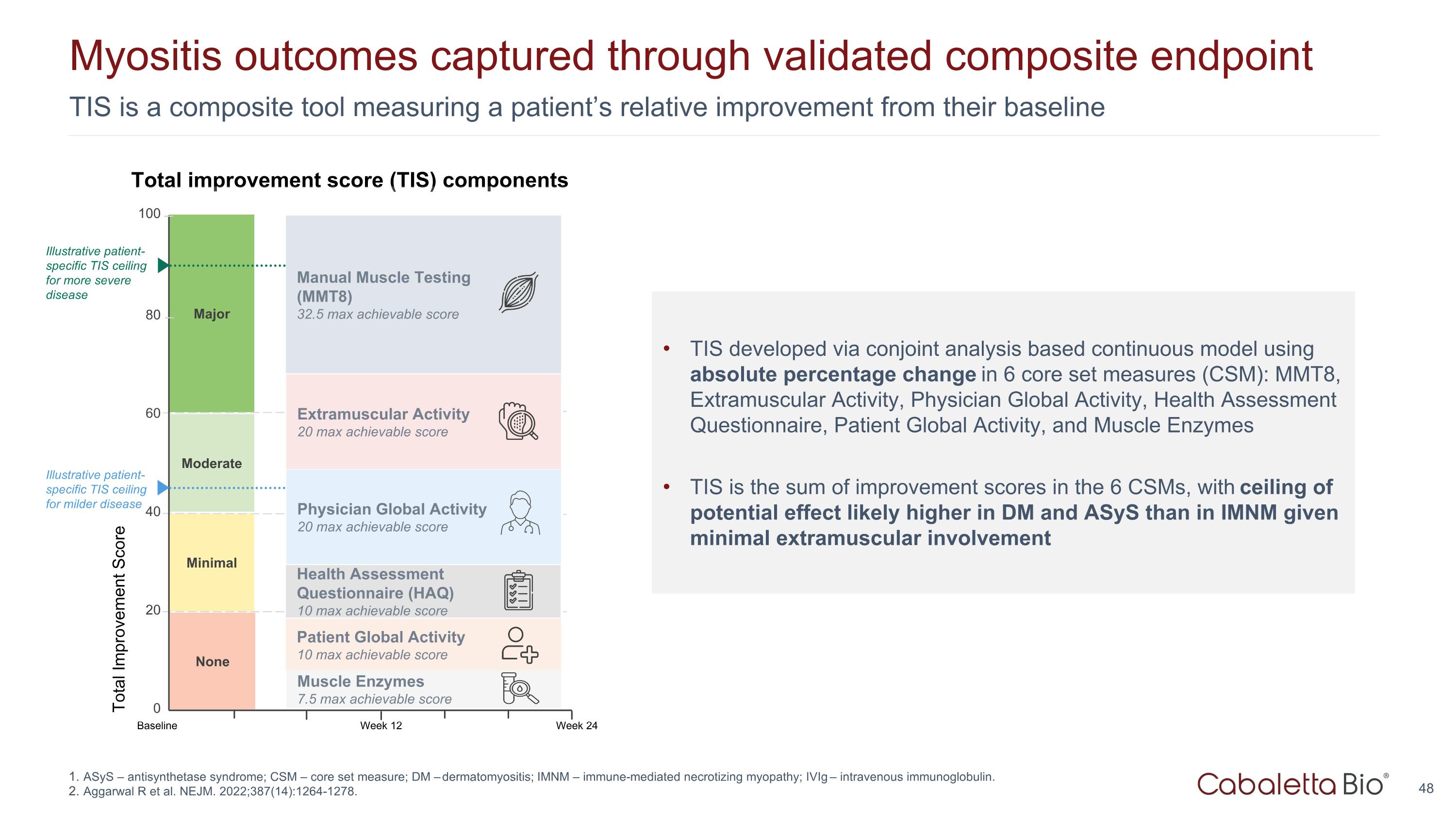
TIS is a composite tool measuring a patient’s relative improvement from their baseline Myositis outcomes captured through validated composite endpoint Major Total Improvement Score 100 80 0 Minimal Moderate 60 40 20 Week 24 Baseline Week 12 None Extramuscular Activity 20 max achievable score Manual Muscle Testing (MMT8) 32.5 max achievable score Patient Global Activity 10 max achievable score Physician Global Activity 20 max achievable score Health Assessment Questionnaire (HAQ) 10 max achievable score Muscle Enzymes 7.5 max achievable score Total improvement score (TIS) components TIS developed via conjoint analysis based continuous model using absolute percentage change in 6 core set measures (CSM): MMT8, Extramuscular Activity, Physician Global Activity, Health Assessment Questionnaire, Patient Global Activity, and Muscle Enzymes TIS is the sum of improvement scores in the 6 CSMs, with ceiling of potential effect likely higher in DM and ASyS than in IMNM given minimal extramuscular involvement Illustrative patient-specific TIS ceiling for milder disease Illustrative patient-specific TIS ceiling for more severe disease ASyS – antisynthetase syndrome; CSM – core set measure; DM – dermatomyositis; IMNM – immune-mediated necrotizing myopathy; IVIg – intravenous immunoglobulin. Aggarwal R et al. NEJM. 2022;387(14):1264-1278.

Corporate Presentation October 2025