

Corporate Presentation October 2025

Disclaimer This presentation, including any printed or electronic copy of these slides, the talks given by the presenters, the information communicated during any delivery of the presentation and any question and answer session and any document distributed at or in connection with the presentation (collectively, the “Presentation”) has been prepared by Cabaletta Bio, Inc. (“we,” “us,” “our,” “Cabaletta” or the “Company”) and may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, and include, but are not limited to, express or implied statements regarding our current beliefs, expectations and assumptions regarding: our business, future plans and strategies for our technology; our ability to grow our autoimmune-focused pipeline; the ability to capitalize on and potential benefits resulting from our research and translational insights, including those related to any similarly-designed constructs or dosing regimens; the anticipated market opportunities for rese-cel in patients with autoimmune diseases; the Company's business plans and objectives; our expectations around the potential success and therapeutic and clinical benefits of rese-cel, as well as our ability to successfully complete research and further development and commercialization of our drug candidates in current or future indications, including the timing and results of our clinical trials and our ability to conduct and complete clinical trials; expectation that clinical results will support rese-cel's safety and activity profile; our plan to leverage increasing clinical data and a unique development program for rese-cel; the timing, clinical significance and impact of clinical data read-outs, including the progress, results and clinical data from each of the patients dosed with rese-cel in the Phase 1/2 RESET-Myositis, RESET-SLE, RESET-SSc, RESET-MG and RESET-PV trials and our other planned activities with respect to rese-cel; our belief that rese-cel has the potential to provide drug-free, durable transformative clinical responses, through an immune reset, including the potential for achieving drug-free remission in patients with refractory myositis; the Company's advancement of separate Phase 1/2 clinical trials of rese-cel and advancement of the RESET-PV and RESET-MS trials, with and without preconditioning, as applicable, including updates related to status, safety data, efficiency of clinical trial design and timing of data read-outs or otherwise; our ability to leverage our experience in autoimmune cell therapy; our ability to enroll the requisite number of patients, dose each dosing cohort in the intended manner and timing thereof, and advance the trial as planned in our Phase 1/2 clinical trials of rese-cel; the timing any planned regulatory filings for our development programs, including IND applications and interactions with regulatory authorities, including such authorities' review of safety information from our ongoing clinical trials and potential registrational pathway for rese-cel in various indications, and the timing of alignment of trial design related thereto; our ability to successfully complete our preclinical and clinical studies for our product candidates, including our ability to progress the trial; Cabaletta's advancement of the whole blood manufacturing program to remove the burden of apheresis; our ability to increase enrollment from our rapidly expanding clinical network in the RESET clinical trial program in the US and Europe; our ability to obtain and maintain regulatory approval of our product candidates, including our expectations regarding the intended incentives conferred by and ability to retain regulatory designations and the anticipated initiation of registrational cohorts and potential BLA submission; our belief regarding alignment with FDA on registrational trial design and timing thereof; our ability to accelerate our pipeline to approval and launch and to develop transformative therapies for patients, including in collaboration with academic and industry partners and the ability to optimize such collaborations on, including timing thereof, our development programs; our ability to contract with third-party suppliers and manufacturers; our ability to execute our manufacturing strategy to enable expansion of clinical supply and efficiently scale commercial supply for rese-cel; our potential commercial opportunities, including value and addressable market, for our product candidates. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Various risks, uncertainties and assumptions could cause actual results to differ materially from those anticipated or implied in our forward-looking statements. Such risks and uncertainties include, but are not limited to, risks related to the success, cost, and timing of our development activities and clinical trials, risks related to our ability to demonstrate sufficient evidence of safety, efficacy and tolerability in our clinical trials, the risk that the results observed with the similarly-designed construct, including, but not limited to, dosing regimen, are not indicative of the results we seek to achieve with rese-cel, the risk that signs of biologic activity or persistence may not inform long-term results, risks related to clinical trial site activation or enrollment rates that are lower than expected, risks that modifications to trial design or approach may not have the intended benefits and that the trial design may need to be further modified; our ability to protect and maintain our intellectual property position, risks related to our relationships with third parties, uncertainties related to regulatory agencies’ evaluation of regulatory filings and other information related to our product candidates, our ability to retain and recognize the intended incentives conferred by any regulatory designations, risks related to regulatory filings and potential clearance, the risk that any one or more of our product candidates will not be successfully developed and commercialized, the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies, risks related to volatile market and economic conditions and our ability to fund operations and continue as a going concern. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ materially from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our most recent annual report on Form 10-K and quarterly report on Form 10-Q, as well as discussions of potential risks, uncertainties, and other important factors in our other filings with the Securities and Exchange Commission. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. The Company is the owner of various trademarks, trade names and service marks. Certain other trademarks, trade names and service marks appearing in this Presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this Presentation are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.

Develop and launch the first curative targeted cellular therapies for patients with autoimmune diseases
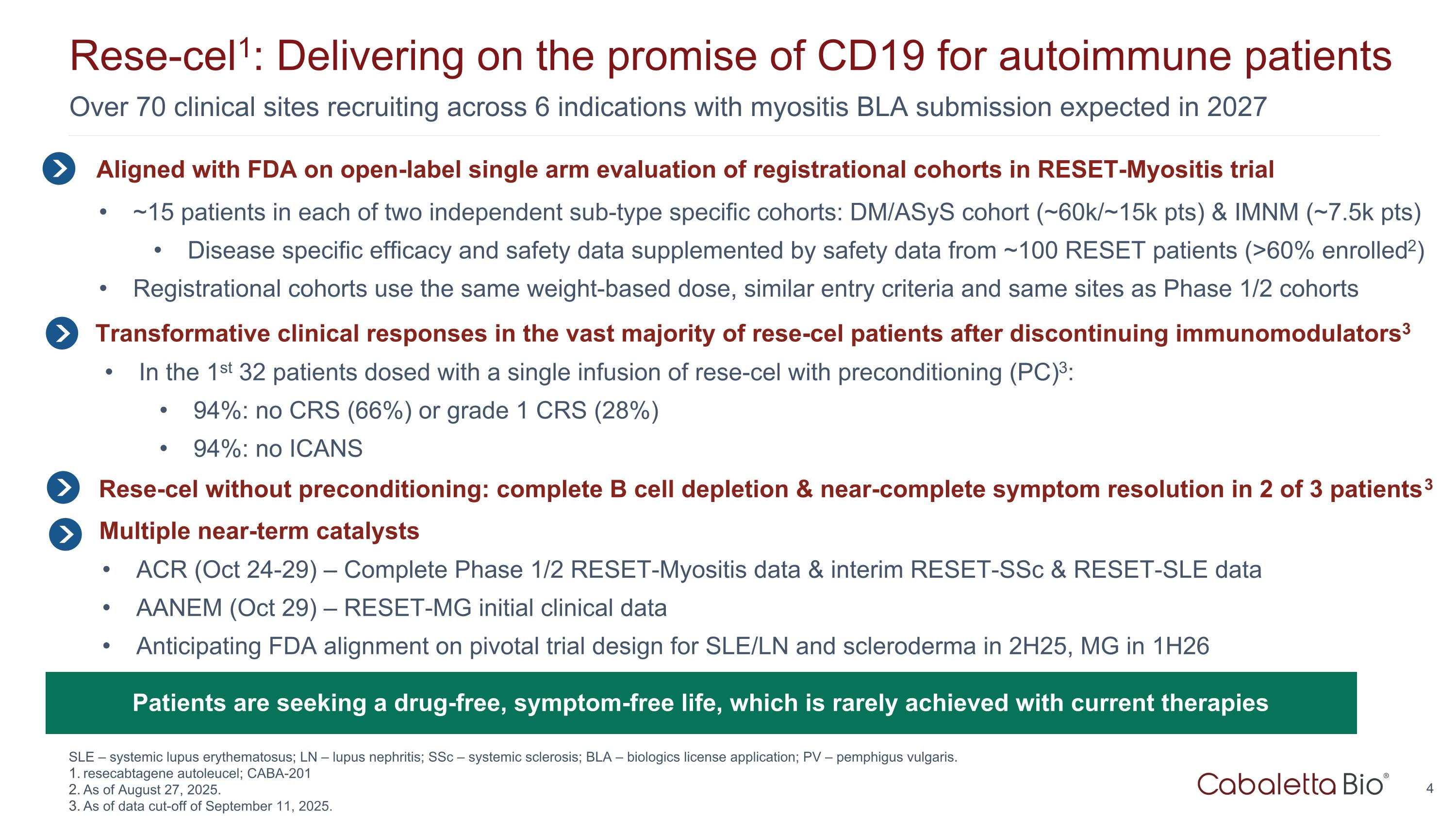
Over 70 clinical sites recruiting across 6 indications with myositis BLA submission expected in 2027 Rese-cel1: Delivering on the promise of CD19 for autoimmune patients SLE – systemic lupus erythematosus; LN – lupus nephritis; SSc – systemic sclerosis; BLA – biologics license application; PV – pemphigus vulgaris. resecabtagene autoleucel; CABA-201 As of August 27, 2025. As of data cut-off of September 11, 2025. Aligned with FDA on open-label single arm evaluation of registrational cohorts in RESET-Myositis trial ~15 patients in each of two independent sub-type specific cohorts: DM/ASyS cohort (~60k/~15k pts) & IMNM (~7.5k pts) Disease specific efficacy and safety data supplemented by safety data from ~100 RESET patients (>60% enrolled2) Registrational cohorts use the same weight-based dose, similar entry criteria and same sites as Phase 1/2 cohorts In the 1st 32 patients dosed with a single infusion of rese-cel with preconditioning (PC)3: 94%: no CRS (66%) or grade 1 CRS (28%) 94%: no ICANS Transformative clinical responses in the vast majority of rese-cel patients after discontinuing immunomodulators3 Patients are seeking a drug-free, symptom-free life, which is rarely achieved with current therapies Multiple near-term catalysts ACR (Oct 24-29) – Complete Phase 1/2 RESET-Myositis data & interim RESET-SSc & RESET-SLE data AANEM (Oct 29) – RESET-MG initial clinical data Anticipating FDA alignment on pivotal trial design for SLE/LN and scleroderma in 2H25, MG in 1H26 Rese-cel without preconditioning: complete B cell depletion & near-complete symptom resolution in 2 of 3 patients3
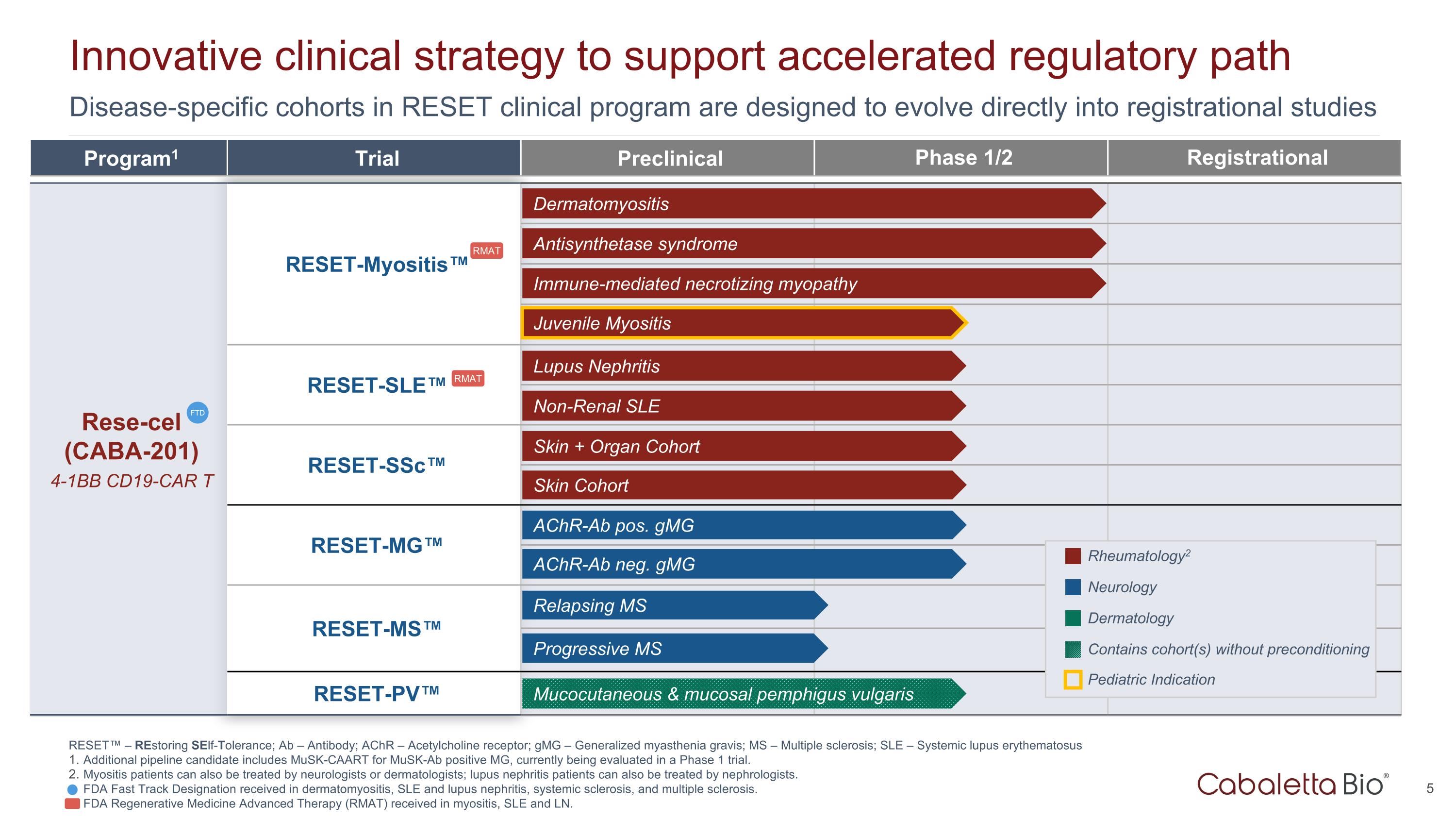
Disease-specific cohorts in RESET clinical program are designed to evolve directly into registrational studies Innovative clinical strategy to support accelerated regulatory path Program1 Trial Preclinical Phase 1/2 Registrational Rese-cel (CABA-201) 4-1BB CD19-CAR T RESET-Myositis™ CARTA Chimeric Antigen Receptor T cells for Autoimmunity RESET-SLE™ RESET-SSc™ RESET-MG™ RESET-MS™ RESET-PV™ Dermatomyositis Antisynthetase syndrome Immune-mediated necrotizing myopathy Lupus Nephritis Non-Renal SLE Skin + Organ Cohort AChR-Ab neg. gMG AChR-Ab pos. gMG Skin Cohort Rheumatology2 Neurology Dermatology FTD Mucocutaneous & mucosal pemphigus vulgaris Contains cohort(s) without preconditioning Juvenile Myositis Pediatric Indication RESET™ – REstoring SElf-Tolerance; Ab – Antibody; AChR – Acetylcholine receptor; gMG – Generalized myasthenia gravis; MS – Multiple sclerosis; SLE – Systemic lupus erythematosus Additional pipeline candidate includes MuSK-CAART for MuSK-Ab positive MG, currently being evaluated in a Phase 1 trial. Myositis patients can also be treated by neurologists or dermatologists; lupus nephritis patients can also be treated by nephrologists. FDA Fast Track Designation received in dermatomyositis, SLE and lupus nephritis, systemic sclerosis, and multiple sclerosis. FDA Regenerative Medicine Advanced Therapy (RMAT) received in myositis, SLE and LN. Progressive MS Relapsing MS RMAT RMAT
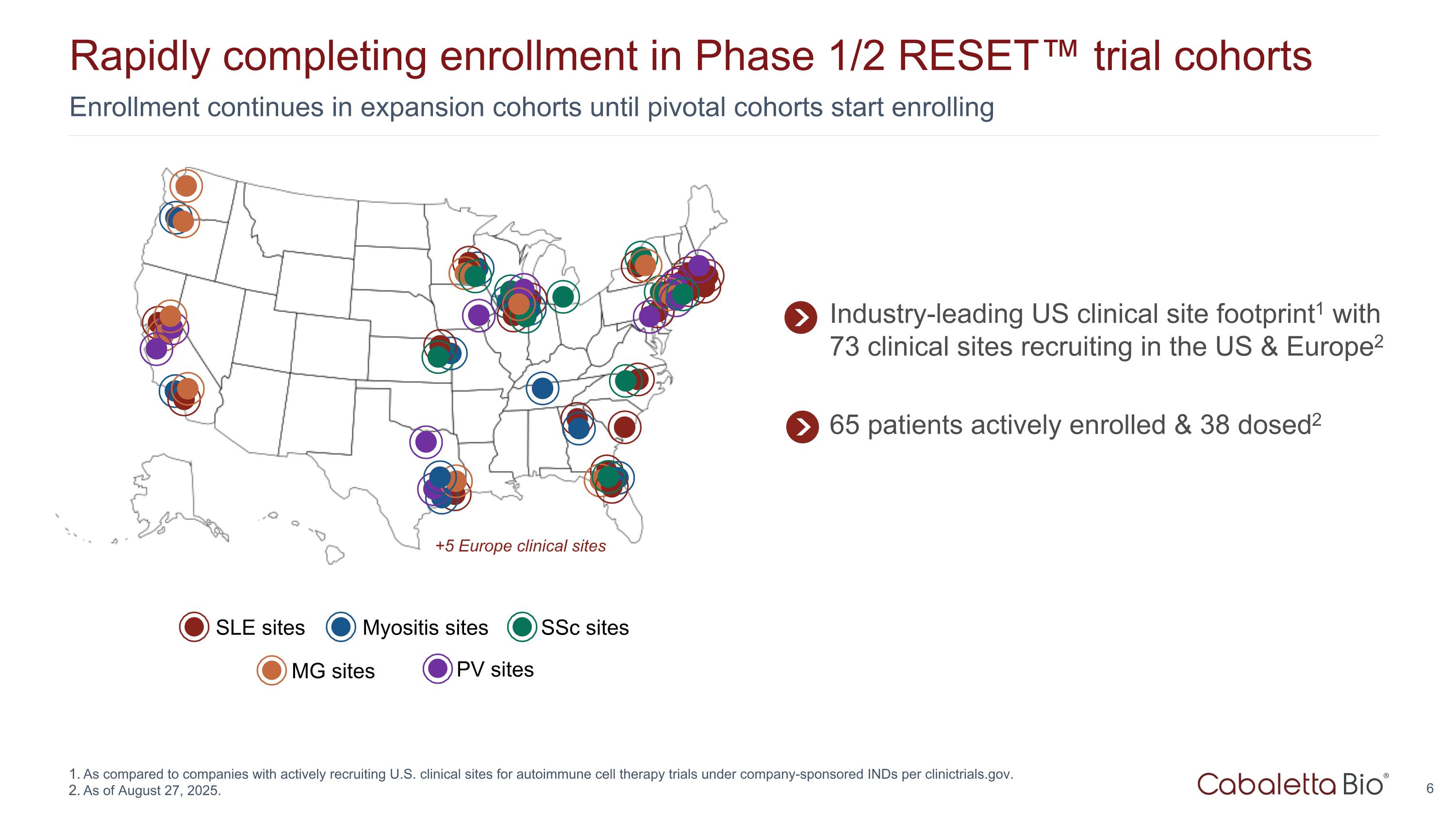
Enrollment continues in expansion cohorts until pivotal cohorts start enrolling Rapidly completing enrollment in Phase 1/2 RESET™ trial cohorts As compared to companies with actively recruiting U.S. clinical sites for autoimmune cell therapy trials under company-sponsored INDs per clinictrials.gov. As of August 27, 2025. SLE sites Myositis sites MG sites SSc sites PV sites +5 Europe clinical sites Industry-leading US clinical site footprint1 with 73 clinical sites recruiting in the US & Europe2 65 patients actively enrolled & 38 dosed2
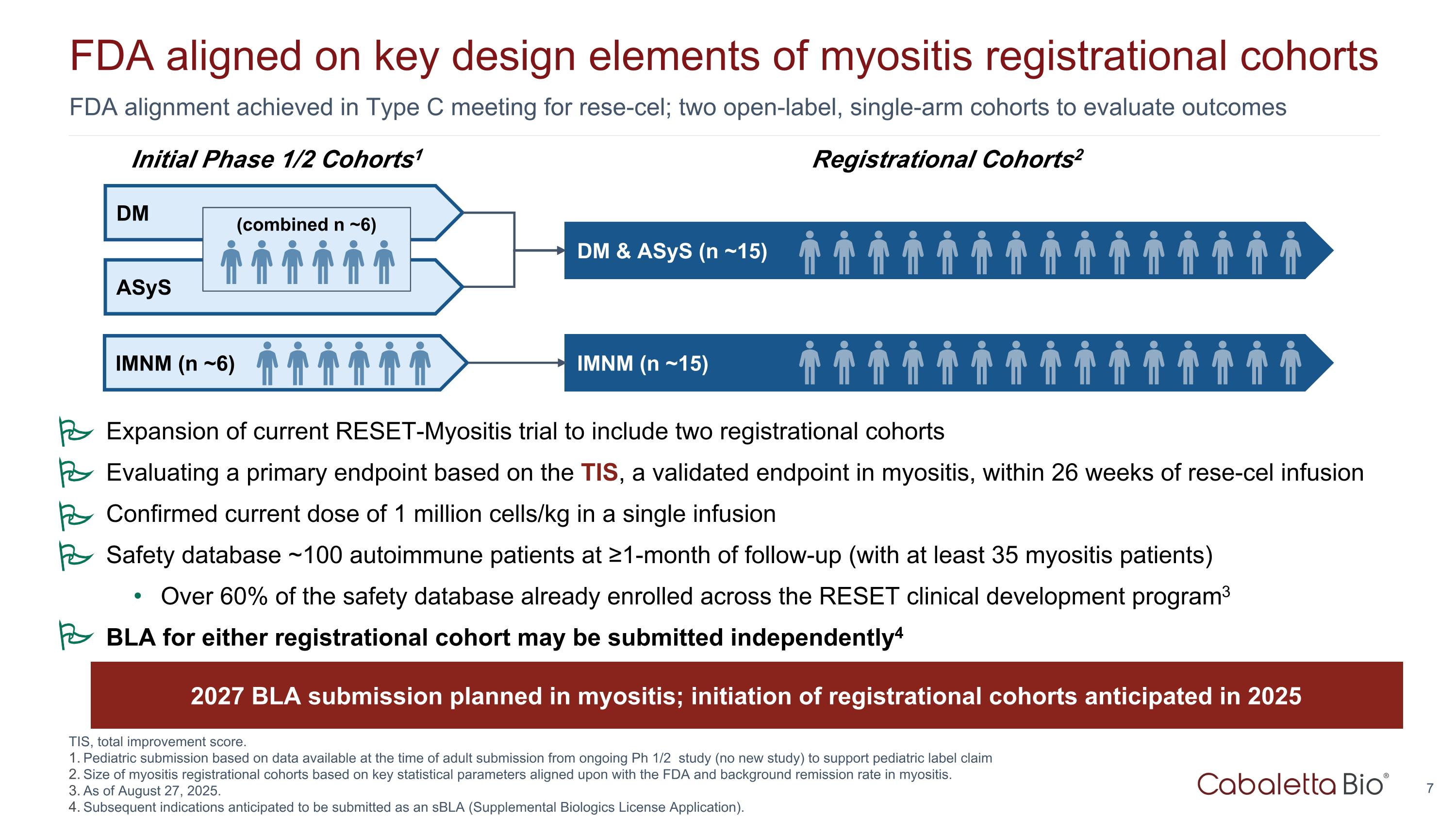
FDA alignment achieved in Type C meeting for rese-cel; two open-label, single-arm cohorts to evaluate outcomes FDA aligned on key design elements of myositis registrational cohorts Registrational Cohorts2 ASyS DM & ASyS (n ~15) Initial Phase 1/2 Cohorts1 DM IMNM (n ~6) IMNM (n ~15) (combined n ~6) TIS, total improvement score. Pediatric submission based on data available at the time of adult submission from ongoing Ph 1/2 study (no new study) to support pediatric label claim Size of myositis registrational cohorts based on key statistical parameters aligned upon with the FDA and background remission rate in myositis. As of August 27, 2025. Subsequent indications anticipated to be submitted as an sBLA (Supplemental Biologics License Application). 2027 BLA submission planned in myositis; initiation of registrational cohorts anticipated in 2025 Expansion of current RESET-Myositis trial to include two registrational cohorts Evaluating a primary endpoint based on the TIS, a validated endpoint in myositis, within 26 weeks of rese-cel infusion Confirmed current dose of 1 million cells/kg in a single infusion Safety database ~100 autoimmune patients at ≥1-month of follow-up (with at least 35 myositis patients) Over 60% of the safety database already enrolled across the RESET clinical development program3 BLA for either registrational cohort may be submitted independently4 P P P P P
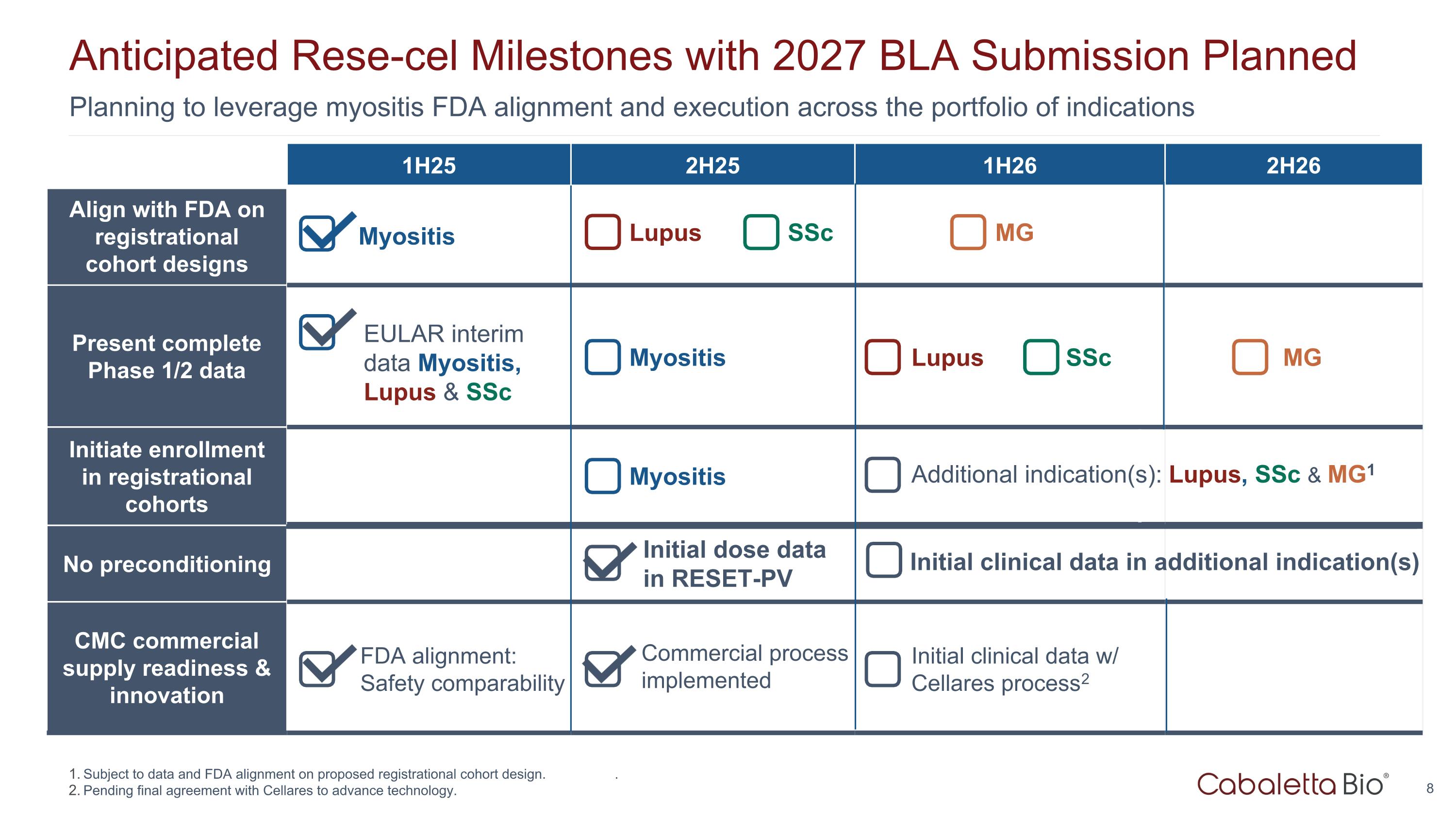
Planning to leverage myositis FDA alignment and execution across the portfolio of indications Anticipated Rese-cel Milestones with 2027 BLA Submission Planned 1H25 2H25 1H26 2H26 Align with FDA on registrational cohort designs Present complete Phase 1/2 data Initiate enrollment in registrational cohorts No preconditioning CMC commercial supply readiness & innovation Initial clinical data in additional indication(s) Initial dose data in RESET-PV Commercial process implemented Lupus SSc Myositis MG Lupus SSc MG Myositis Myositis FDA alignment: Safety comparability EULAR interim data Myositis, Lupus & SSc Initial clinical data w/ Cellares process2 Subject to data and FDA alignment on proposed registrational cohort design. . Pending final agreement with Cellares to advance technology. Additional indication(s): Lupus, SSc & MG1

Rese-cel: Autologus CD19-CAR T for Autoimmunity
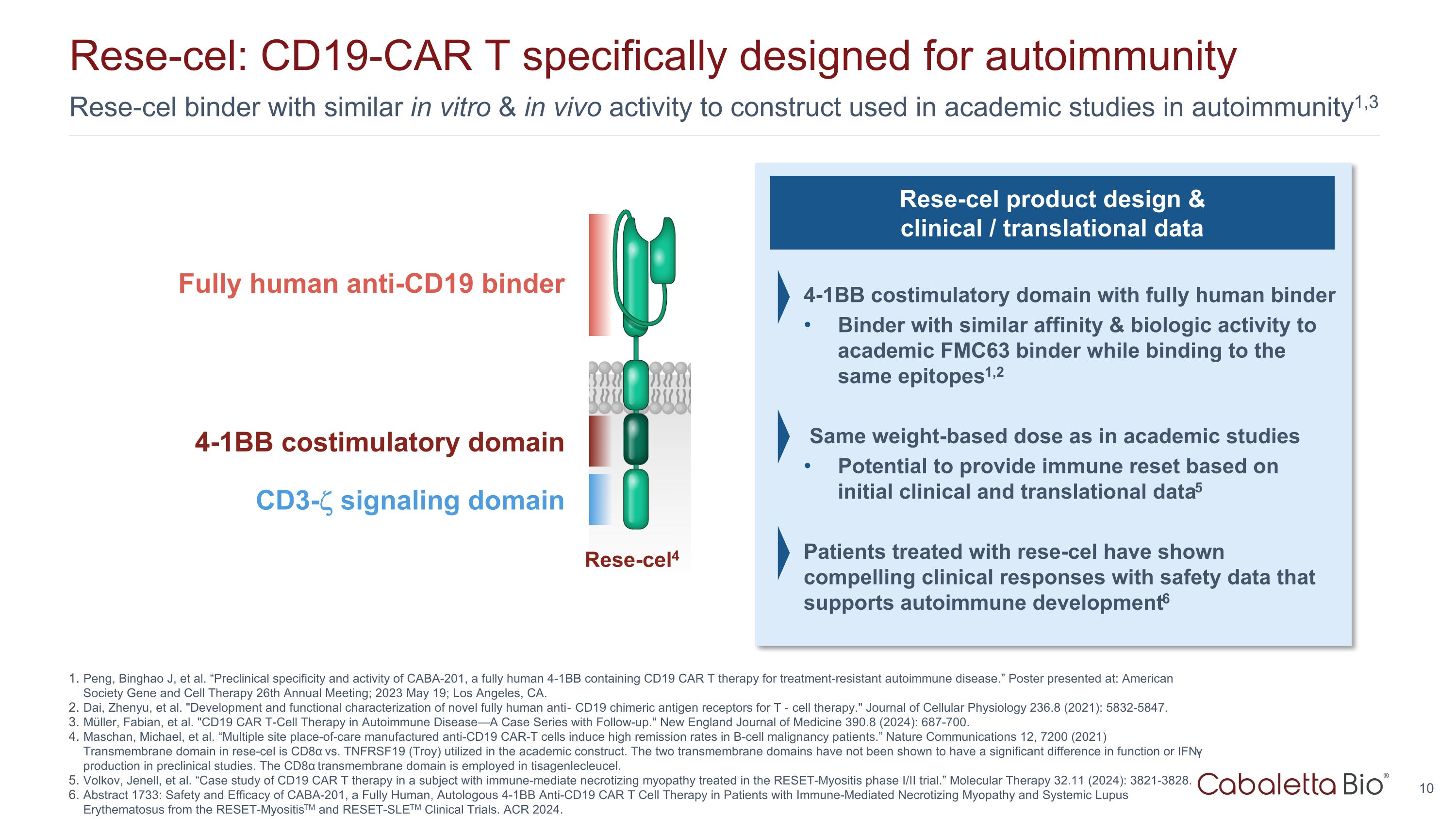
Rese-cel binder with similar in vitro & in vivo activity to construct used in academic studies in autoimmunity1,3 Rese-cel: CD19-CAR T specifically designed for autoimmunity Peng, Binghao J, et al. “Preclinical specificity and activity of CABA-201, a fully human 4-1BB containing CD19 CAR T therapy for treatment-resistant autoimmune disease.” Poster presented at: American Society Gene and Cell Therapy 26th Annual Meeting; 2023 May 19; Los Angeles, CA. Dai, Zhenyu, et al. "Development and functional characterization of novel fully human anti‐CD19 chimeric antigen receptors for T‐cell therapy." Journal of Cellular Physiology 236.8 (2021): 5832-5847. Müller, Fabian, et al. "CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up." New England Journal of Medicine 390.8 (2024): 687-700. Maschan, Michael, et al. “Multiple site place-of-care manufactured anti-CD19 CAR-T cells induce high remission rates in B-cell malignancy patients.” Nature Communications 12, 7200 (2021) Transmembrane domain in rese-cel is CD8α vs. TNFRSF19 (Troy) utilized in the academic construct. The two transmembrane domains have not been shown to have a significant difference in function or IFN-γ production in preclinical studies. The CD8α transmembrane domain is employed in tisagenlecleucel. Volkov, Jenell, et al. “Case study of CD19 CAR T therapy in a subject with immune-mediate necrotizing myopathy treated in the RESET-Myositis phase I/II trial.” Molecular Therapy 32.11 (2024): 3821-3828. Abstract 1733: Safety and Efficacy of CABA-201, a Fully Human, Autologous 4-1BB Anti-CD19 CAR T Cell Therapy in Patients with Immune-Mediated Necrotizing Myopathy and Systemic Lupus Erythematosus from the RESET-MyositisTM and RESET-SLETM Clinical Trials. ACR 2024. Rese-cel product design & clinical / translational data 4-1BB costimulatory domain with fully human binder Binder with similar affinity & biologic activity to academic FMC63 binder while binding to the same epitopes1,2 Same weight-based dose as in academic studies Potential to provide immune reset based on initial clinical and translational data5 Patients treated with rese-cel have shown compelling clinical responses with safety data that supports autoimmune development6 Fully human anti-CD19 binder 4-1BB costimulatory domain CD3- signaling domain Rese-cel4
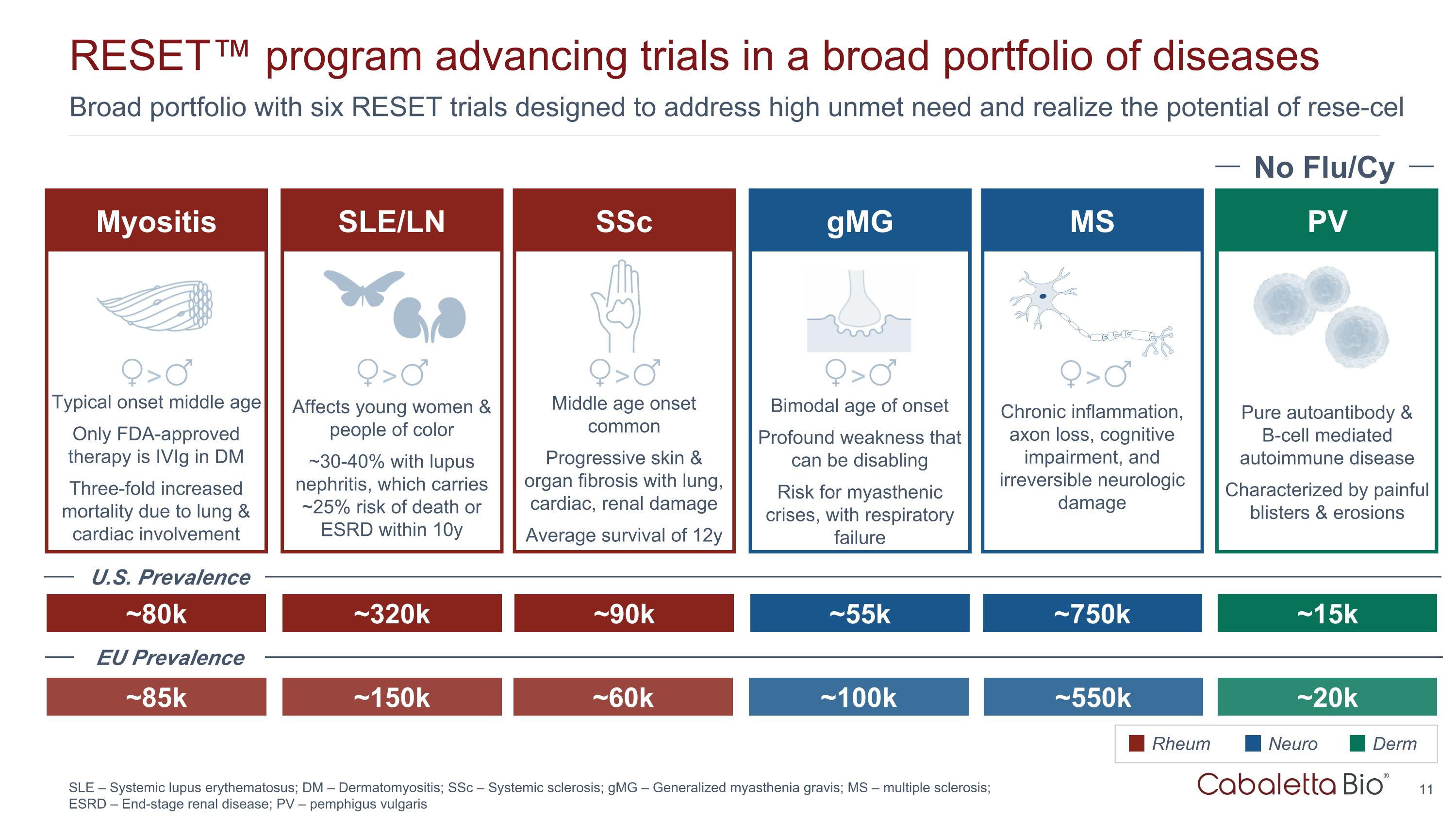
Broad portfolio with six RESET trials designed to address high unmet need and realize the potential of rese-cel RESET™ program advancing trials in a broad portfolio of diseases SLE – Systemic lupus erythematosus; DM – Dermatomyositis; SSc – Systemic sclerosis; gMG – Generalized myasthenia gravis; MS – multiple sclerosis; ESRD – End-stage renal disease; PV – pemphigus vulgaris SSc gMG PV ~90k ~55k ~15k Middle age onset common Progressive skin & organ fibrosis with lung, cardiac, renal damage Average survival of 12y Bimodal age of onset Profound weakness that can be disabling Risk for myasthenic crises, with respiratory failure Rheum Neuro Derm No Flu/Cy Pure autoantibody & B-cell mediated autoimmune disease Characterized by painful blisters & erosions SLE/LN ~320k Affects young women & people of color ~30-40% with lupus nephritis, which carries ~25% risk of death or ESRD within 10y > > > Myositis ~80k Typical onset middle age Only FDA-approved therapy is IVIg in DM Three-fold increased mortality due to lung & cardiac involvement > MS ~750k Chronic inflammation, axon loss, cognitive impairment, and irreversible neurologic damage > ~85k ~150k ~60k ~100k ~550k ~20k U.S. Prevalence EU Prevalence
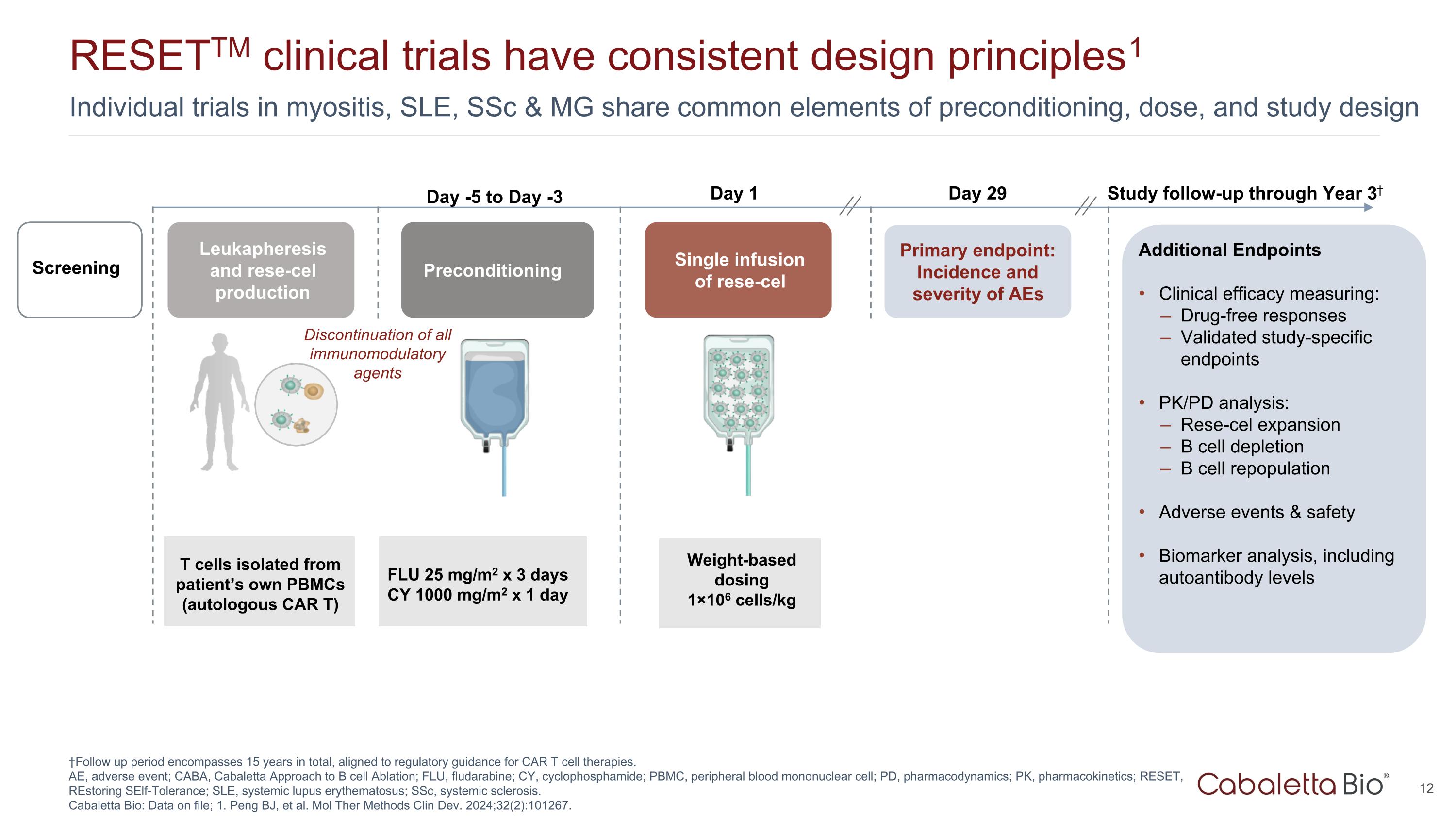
Individual trials in myositis, SLE, SSc & MG share common elements of preconditioning, dose, and study design RESETTM clinical trials have consistent design principles1 †Follow up period encompasses 15 years in total, aligned to regulatory guidance for CAR T cell therapies. AE, adverse event; CABA, Cabaletta Approach to B cell Ablation; FLU, fludarabine; CY, cyclophosphamide; PBMC, peripheral blood mononuclear cell; PD, pharmacodynamics; PK, pharmacokinetics; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus; SSc, systemic sclerosis. Cabaletta Bio: Data on file; 1. Peng BJ, et al. Mol Ther Methods Clin Dev. 2024;32(2):101267. Leukapheresis and rese-cel production Preconditioning Single infusion of rese-cel Weight-based dosing 1×106 cells/kg Day 1 Primary endpoint: Incidence and severity of AEs T cells isolated from patient’s own PBMCs (autologous CAR T) Day 29 Study follow-up through Year 3† Screening Additional Endpoints Clinical efficacy measuring: Drug-free responses Validated study-specific endpoints PK/PD analysis: Rese-cel expansion B cell depletion B cell repopulation Adverse events & safety Biomarker analysis, including autoantibody levels FLU 25 mg/m2 x 3 days CY 1000 mg/m2 x 1 day Day -5 to Day -3 Discontinuation of all immunomodulatory agents
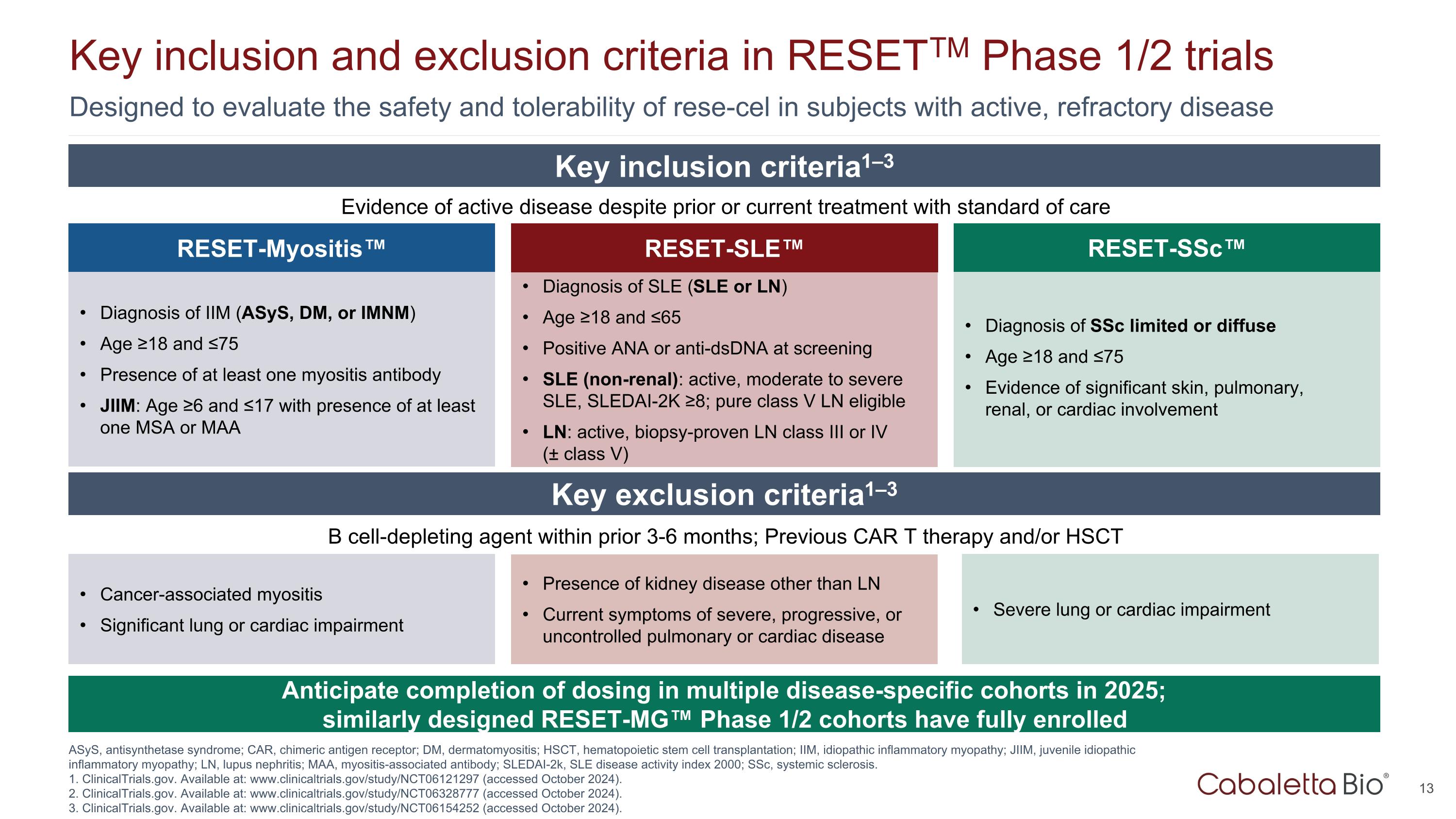
Designed to evaluate the safety and tolerability of rese-cel in subjects with active, refractory disease Key inclusion and exclusion criteria in RESETTM Phase 1/2 trials ASyS, antisynthetase syndrome; CAR, chimeric antigen receptor; DM, dermatomyositis; HSCT, hematopoietic stem cell transplantation; IIM, idiopathic inflammatory myopathy; JIIM, juvenile idiopathic inflammatory myopathy; LN, lupus nephritis; MAA, myositis-associated antibody; SLEDAI-2k, SLE disease activity index 2000; SSc, systemic sclerosis. 1. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06121297 (accessed October 2024). 2. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06328777 (accessed October 2024). 3. ClinicalTrials.gov. Available at: www.clinicaltrials.gov/study/NCT06154252 (accessed October 2024). RESET-SLE™ Diagnosis of SLE (SLE or LN) Age ≥18 and ≤65 Positive ANA or anti-dsDNA at screening SLE (non-renal): active, moderate to severe SLE, SLEDAI-2K ≥8; pure class V LN eligible LN: active, biopsy-proven LN class III or IV (± class V) RESET-Myositis™ Diagnosis of IIM (ASyS, DM, or IMNM) Age ≥18 and ≤75 Presence of at least one myositis antibody JIIM: Age ≥6 and ≤17 with presence of at least one MSA or MAA Diagnosis of SSc limited or diffuse Age ≥18 and ≤75 Evidence of significant skin, pulmonary, renal, or cardiac involvement RESET-SSc™ Evidence of active disease despite prior or current treatment with standard of care Key inclusion criteria1–3 Key exclusion criteria1–3 B cell-depleting agent within prior 3-6 months; Previous CAR T therapy and/or HSCT Presence of kidney disease other than LN Current symptoms of severe, progressive, or uncontrolled pulmonary or cardiac disease Cancer-associated myositis Significant lung or cardiac impairment Severe lung or cardiac impairment Anticipate completion of dosing in multiple disease-specific cohorts in 2025; similarly designed RESET-MG™ Phase 1/2 cohorts have fully enrolled
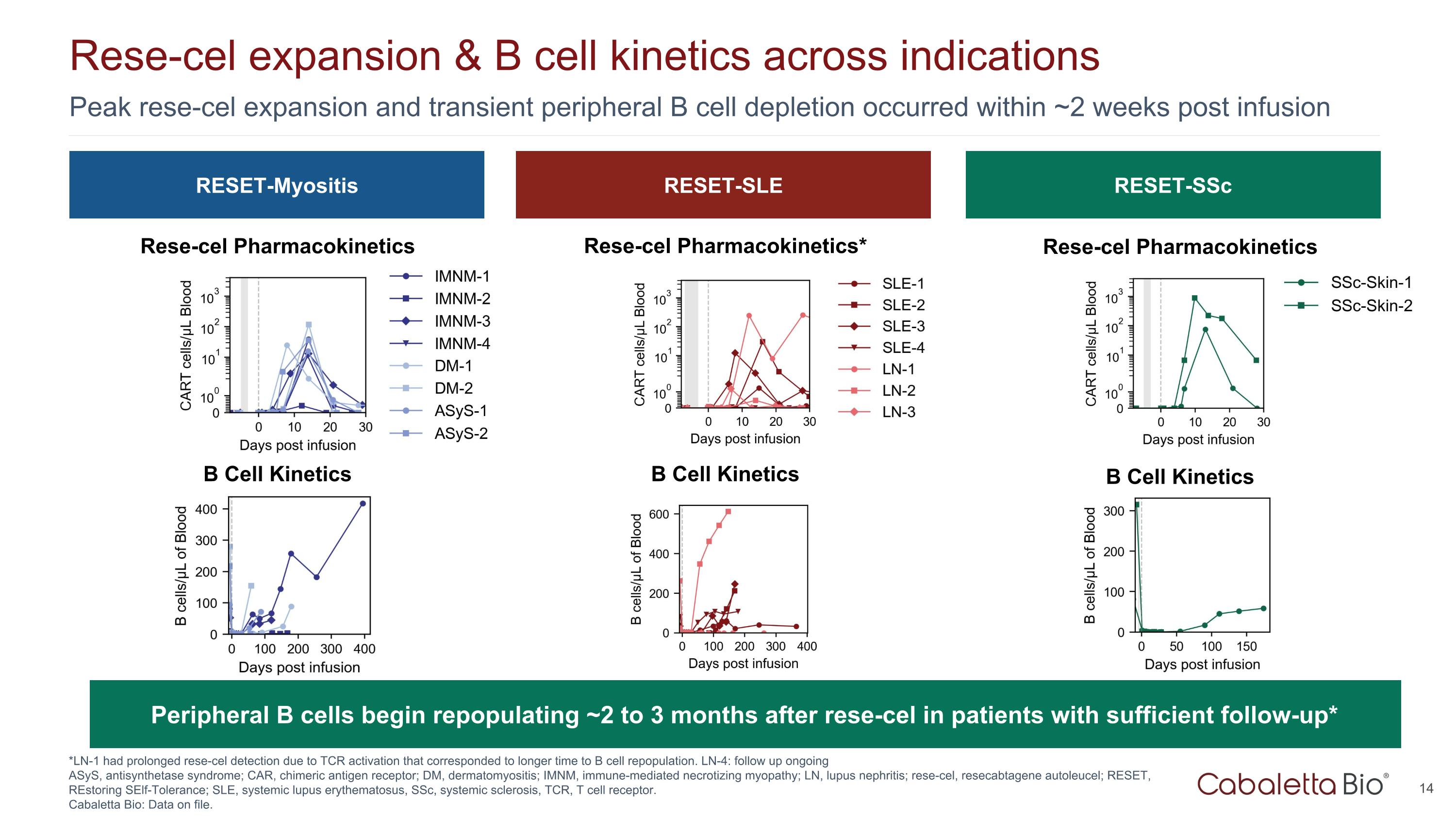
Peak rese-cel expansion and transient peripheral B cell depletion occurred within ~2 weeks post infusion Rese-cel expansion & B cell kinetics across indications *LN-1 had prolonged rese-cel detection due to TCR activation that corresponded to longer time to B cell repopulation. LN-4: follow up ongoing ASyS, antisynthetase syndrome; CAR, chimeric antigen receptor; DM, dermatomyositis; IMNM, immune-mediated necrotizing myopathy; LN, lupus nephritis; rese-cel, resecabtagene autoleucel; RESET, REstoring SElf-Tolerance; SLE, systemic lupus erythematosus, SSc, systemic sclerosis, TCR, T cell receptor. Cabaletta Bio: Data on file. RESET-Myositis RESET-SLE RESET-SSc Rese-cel Pharmacokinetics Rese-cel Pharmacokinetics* Rese-cel Pharmacokinetics B Cell Kinetics B Cell Kinetics B Cell Kinetics Peripheral B cells begin repopulating ~2 to 3 months after rese-cel in patients with sufficient follow-up*

Myositis: Unmet Need & Clinical Data
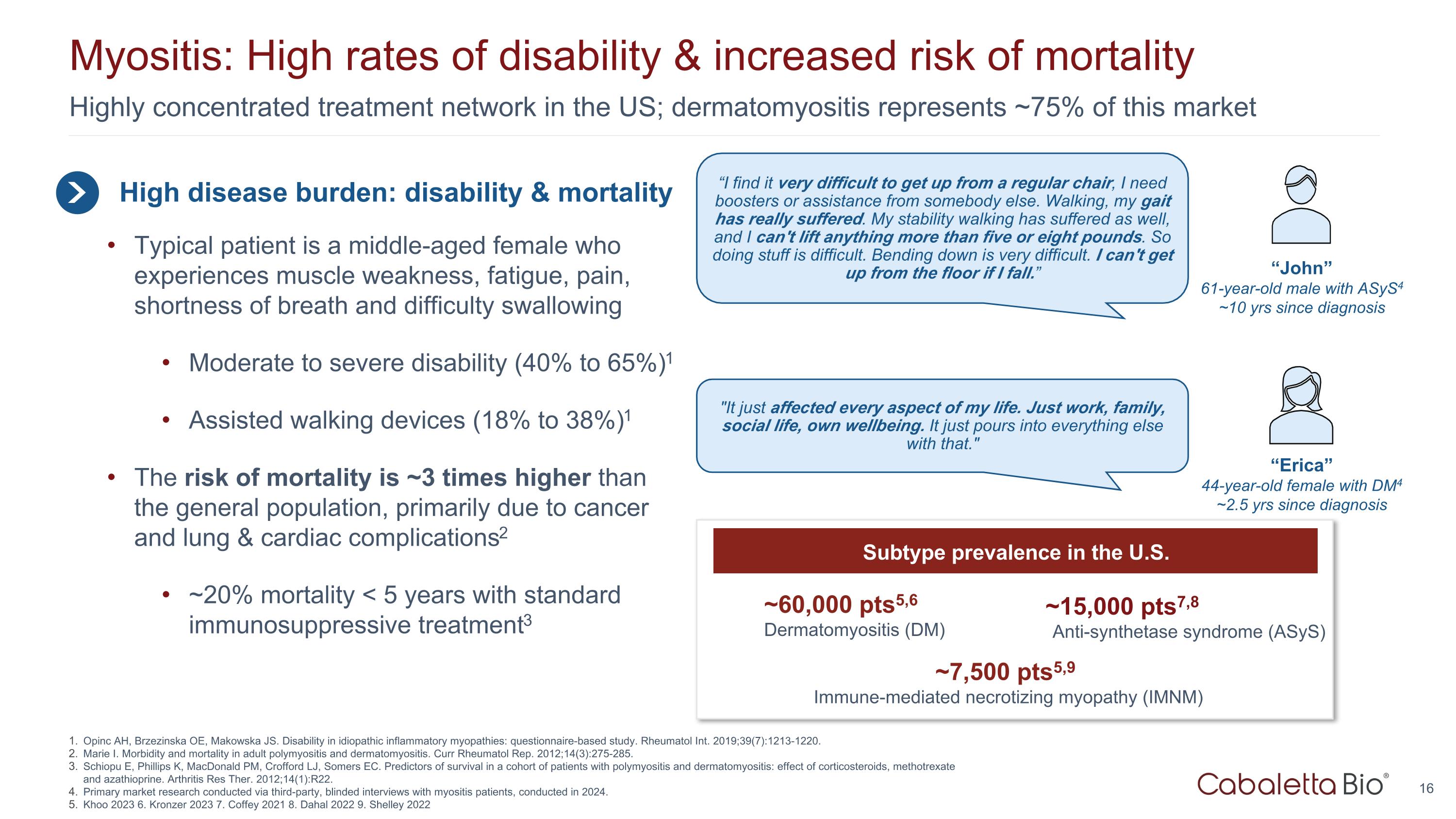
Highly concentrated treatment network in the US; dermatomyositis represents ~75% of this market Myositis: High rates of disability & increased risk of mortality High disease burden: disability & mortality Typical patient is a middle-aged female who experiences muscle weakness, fatigue, pain, shortness of breath and difficulty swallowing Moderate to severe disability (40% to 65%)1 Assisted walking devices (18% to 38%)1 The risk of mortality is ~3 times higher than the general population, primarily due to cancer and lung & cardiac complications2 ~20% mortality < 5 years with standard immunosuppressive treatment3 “I find it very difficult to get up from a regular chair, I need boosters or assistance from somebody else. Walking, my gait has really suffered. My stability walking has suffered as well, and I can't lift anything more than five or eight pounds. So doing stuff is difficult. Bending down is very difficult. I can't get up from the floor if I fall.” “John” 61-year-old male with ASyS4 ~10 yrs since diagnosis "It just affected every aspect of my life. Just work, family, social life, own wellbeing. It just pours into everything else with that." “Erica” 44-year-old female with DM4 ~2.5 yrs since diagnosis Opinc AH, Brzezinska OE, Makowska JS. Disability in idiopathic inflammatory myopathies: questionnaire-based study. Rheumatol Int. 2019;39(7):1213-1220. Marie I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep. 2012;14(3):275-285. Schiopu E, Phillips K, MacDonald PM, Crofford LJ, Somers EC. Predictors of survival in a cohort of patients with polymyositis and dermatomyositis: effect of corticosteroids, methotrexate and azathioprine. Arthritis Res Ther. 2012;14(1):R22. Primary market research conducted via third-party, blinded interviews with myositis patients, conducted in 2024. Khoo 2023 6. Kronzer 2023 7. Coffey 2021 8. Dahal 2022 9. Shelley 2022 ~7,500 pts5,9 Immune-mediated necrotizing myopathy (IMNM) ~15,000 pts7,8 Anti-synthetase syndrome (ASyS) ~60,000 pts5,6 Dermatomyositis (DM) Subtype prevalence in the U.S.
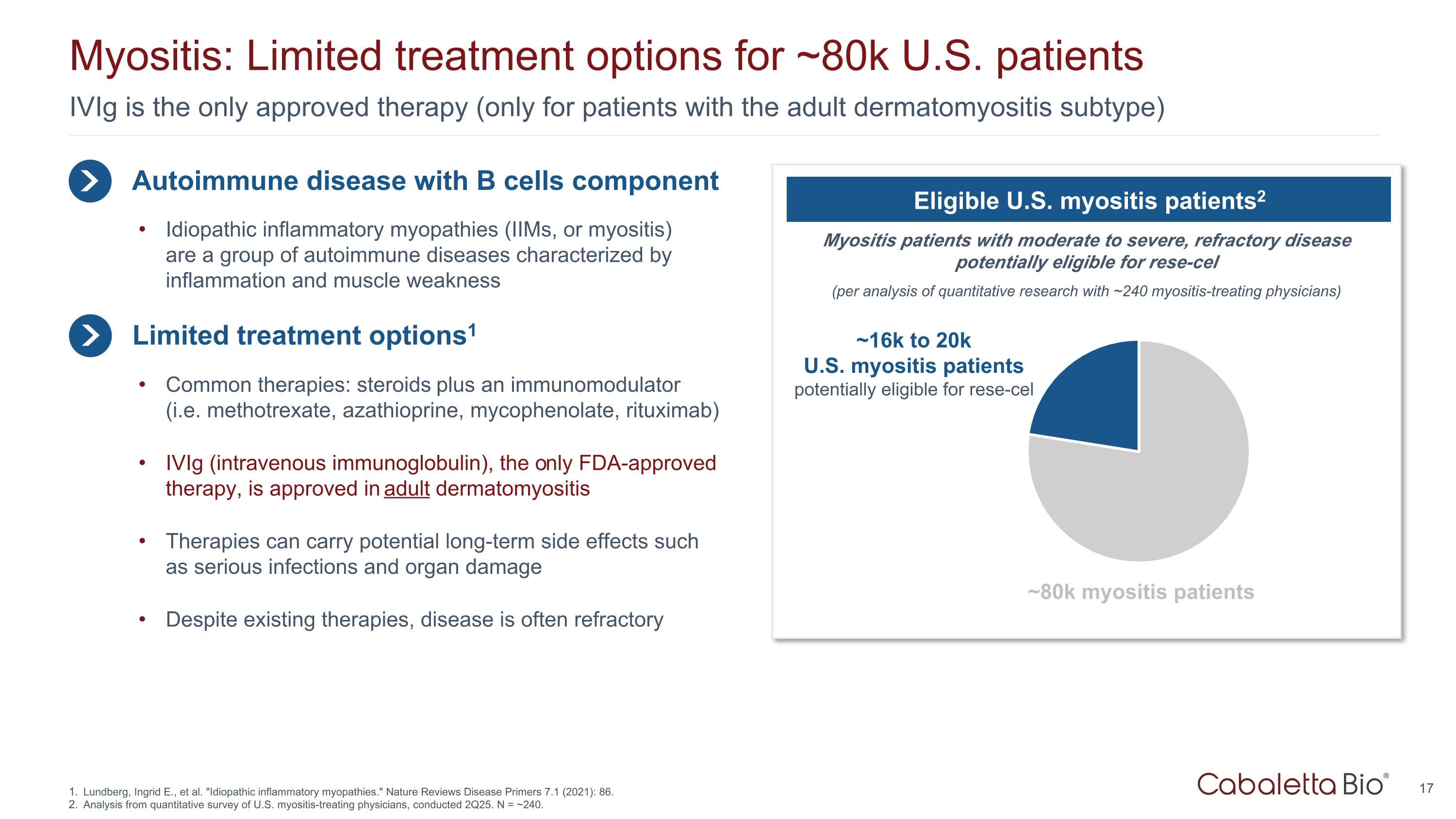
IVIg is the only approved therapy (only for patients with the adult dermatomyositis subtype) Myositis: Limited treatment options for ~80k U.S. patients Lundberg, Ingrid E., et al. "Idiopathic inflammatory myopathies." Nature Reviews Disease Primers 7.1 (2021): 86. Analysis from quantitative survey of U.S. myositis-treating physicians, conducted 2Q25. N = ~240. Autoimmune disease with B cells component Limited treatment options1 Idiopathic inflammatory myopathies (IIMs, or myositis) are a group of autoimmune diseases characterized by inflammation and muscle weakness Common therapies: steroids plus an immunomodulator (i.e. methotrexate, azathioprine, mycophenolate, rituximab) IVIg (intravenous immunoglobulin), the only FDA-approved therapy, is approved in adult dermatomyositis Therapies can carry potential long-term side effects such as serious infections and organ damage Despite existing therapies, disease is often refractory Myositis patients with moderate to severe, refractory disease potentially eligible for rese-cel (per analysis of quantitative research with ~240 myositis-treating physicians) Eligible U.S. myositis patients2 ~16k to 20k U.S. myositis patients potentially eligible for rese-cel ~80k myositis patients
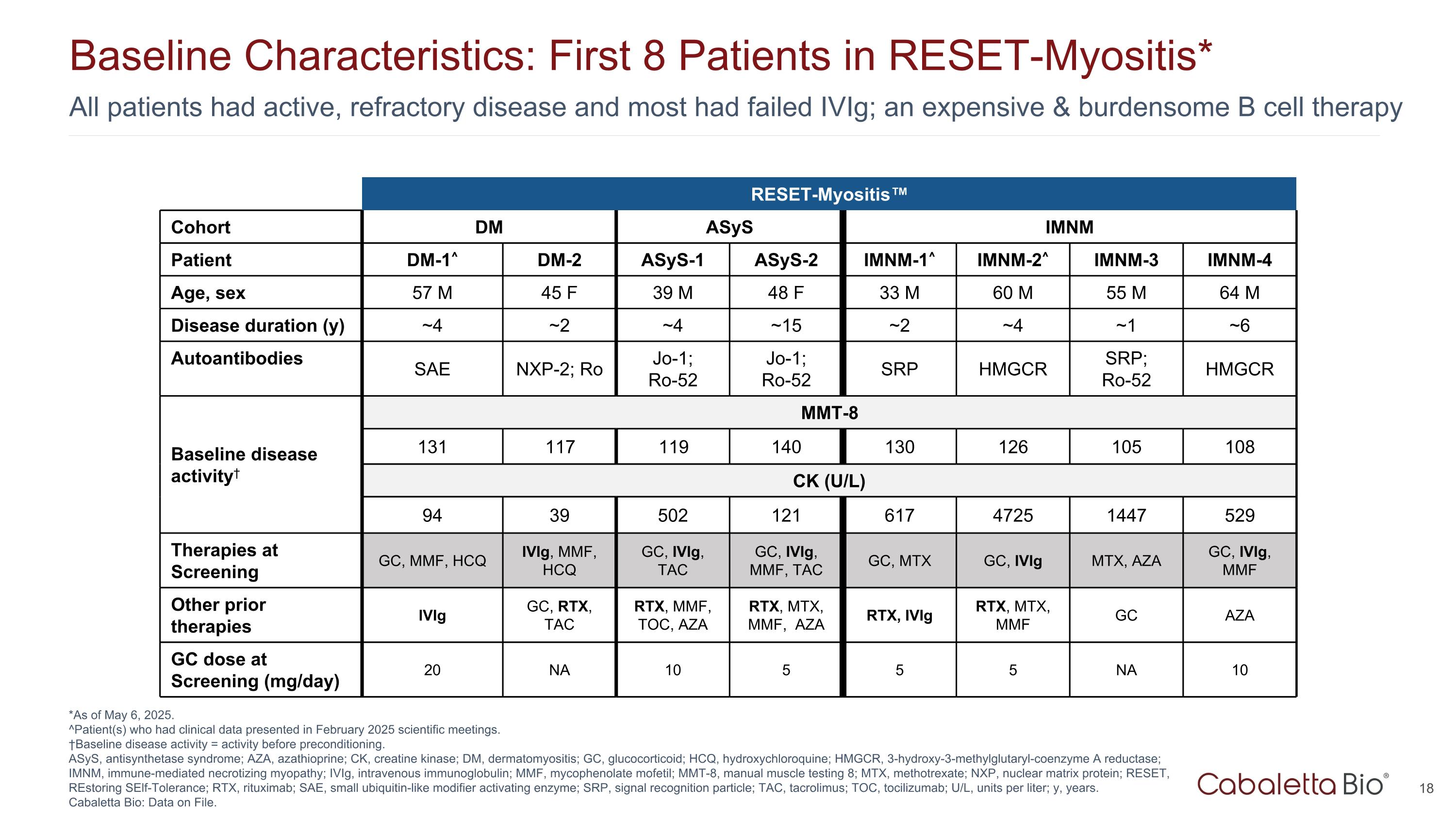
All patients had active, refractory disease and most had failed IVIg; an expensive & burdensome B cell therapy Baseline Characteristics: First 8 Patients in RESET-Myositis* *As of May 6, 2025. ^Patient(s) who had clinical data presented in February 2025 scientific meetings. †Baseline disease activity = activity before preconditioning. ASyS, antisynthetase syndrome; AZA, azathioprine; CK, creatine kinase; DM, dermatomyositis; GC, glucocorticoid; HCQ, hydroxychloroquine; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IMNM, immune-mediated necrotizing myopathy; IVIg, intravenous immunoglobulin; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; MTX, methotrexate; NXP, nuclear matrix protein; RESET, REstoring SElf-Tolerance; RTX, rituximab; SAE, small ubiquitin-like modifier activating enzyme; SRP, signal recognition particle; TAC, tacrolimus; TOC, tocilizumab; U/L, units per liter; y, years. Cabaletta Bio: Data on File. RESET-Myositis™ RESET-Myositis Cohort DM ASyS IMNM Patient DM-1^ DM-2 ASyS-1 ASyS-2 IMNM-1^ IMNM-2^ IMNM-3 IMNM-4 Age, sex 57 M 45 F 39 M 48 F 33 M 60 M 55 M 64 M Disease duration (y) ~4 ~2 ~4 ~15 ~2 ~4 ~1 ~6 Autoantibodies SAE NXP-2; Ro Jo-1; Ro-52 Jo-1; Ro-52 SRP HMGCR SRP; Ro-52 HMGCR Baseline disease activity† MMT-8 131 117 119 140 130 126 105 108 CK (U/L) 94 39 502 121 617 4725 1447 529 Therapies at Screening GC, MMF, HCQ IVIg, MMF, HCQ GC, IVIg, TAC GC, IVIg, MMF, TAC GC, MTX GC, IVIg MTX, AZA GC, IVIg, MMF Other prior therapies IVIg GC, RTX, TAC RTX, MMF, TOC, AZA RTX, MTX, MMF, AZA RTX, IVIg RTX, MTX, MMF GC AZA GC dose at Screening (mg/day) 20 NA 10 5 5 5 NA 10
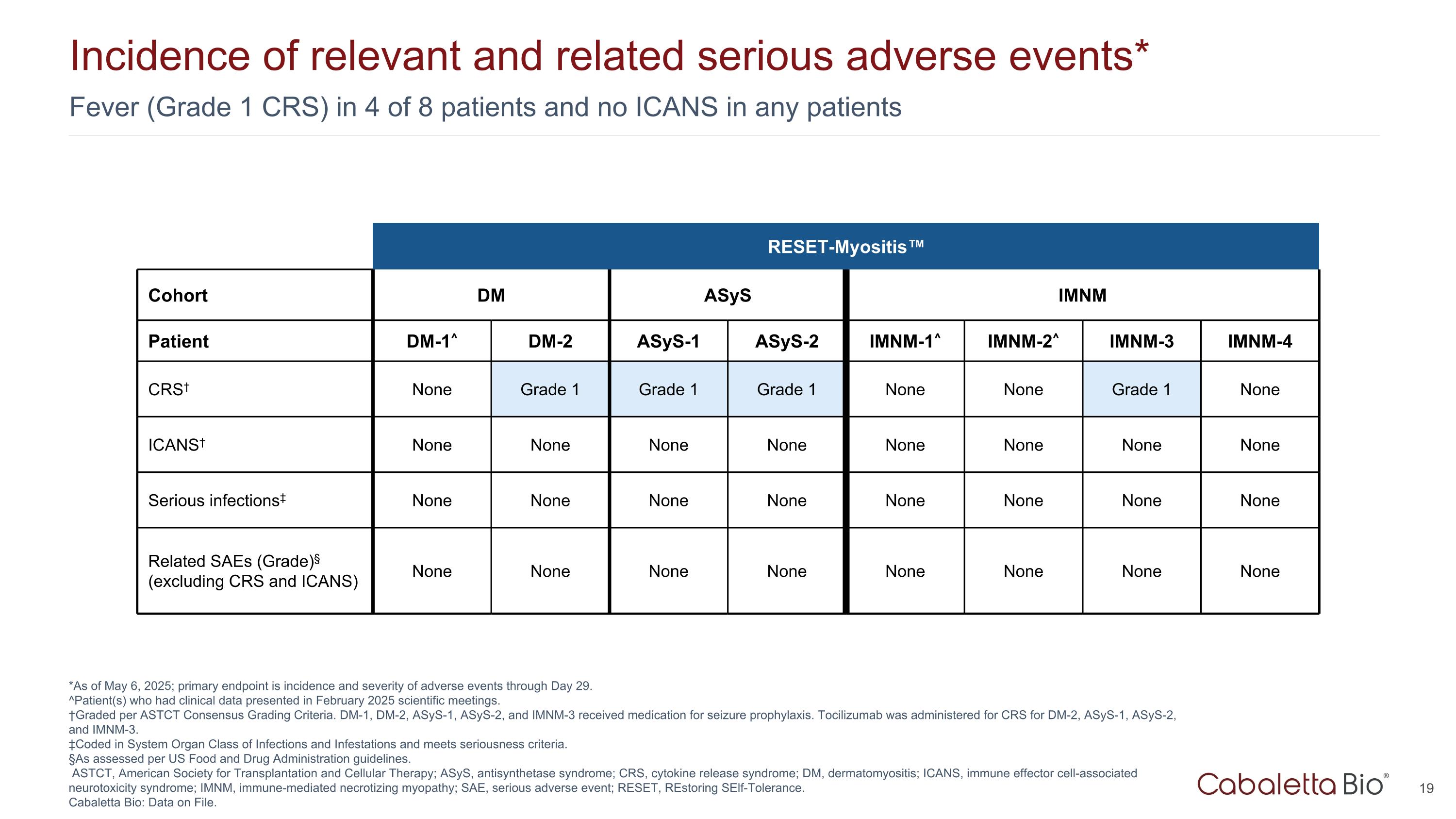
Fever (Grade 1 CRS) in 4 of 8 patients and no ICANS in any patients Incidence of relevant and related serious adverse events* *As of May 6, 2025; primary endpoint is incidence and severity of adverse events through Day 29. ^Patient(s) who had clinical data presented in February 2025 scientific meetings. †Graded per ASTCT Consensus Grading Criteria. DM-1, DM-2, ASyS-1, ASyS-2, and IMNM-3 received medication for seizure prophylaxis. Tocilizumab was administered for CRS for DM-2, ASyS-1, ASyS-2, and IMNM-3. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per US Food and Drug Administration guidelines. ASTCT, American Society for Transplantation and Cellular Therapy; ASyS, antisynthetase syndrome; CRS, cytokine release syndrome; DM, dermatomyositis; ICANS, immune effector cell-associated neurotoxicity syndrome; IMNM, immune-mediated necrotizing myopathy; SAE, serious adverse event; RESET, REstoring SElf-Tolerance. Cabaletta Bio: Data on File. RESET-Myositis™ Cohort DM ASyS IMNM Patient DM-1^ DM-2 ASyS-1 ASyS-2 IMNM-1^ IMNM-2^ IMNM-3 IMNM-4 CRS† None Grade 1 Grade 1 Grade 1 None None Grade 1 None ICANS† None None None None None None None None Serious infections‡ None None None None None None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None None None None None None
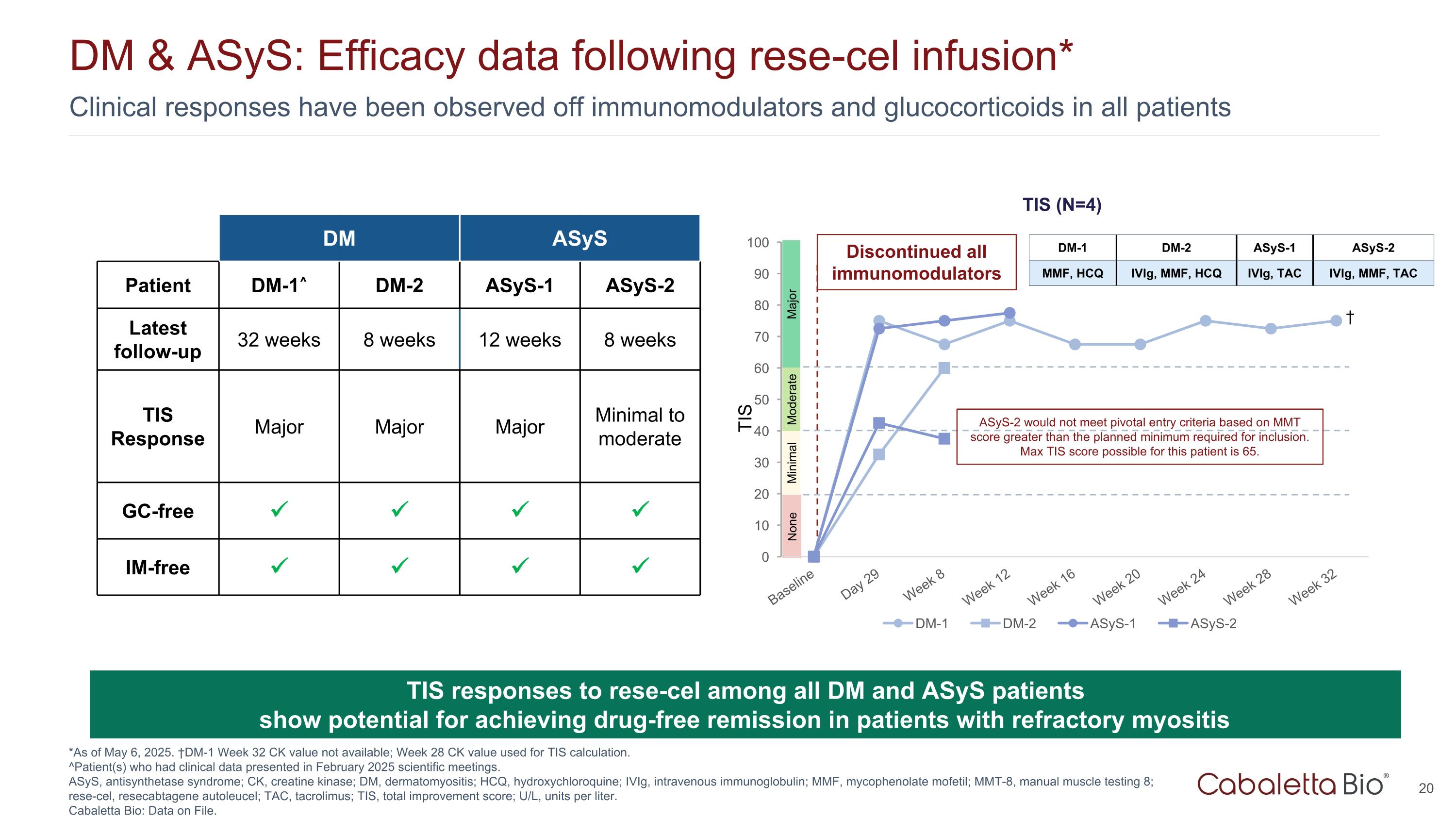
Clinical responses have been observed off immunomodulators and glucocorticoids in all patients DM & ASyS: Efficacy data following rese-cel infusion* *As of May 6, 2025. †DM-1 Week 32 CK value not available; Week 28 CK value used for TIS calculation. ^Patient(s) who had clinical data presented in February 2025 scientific meetings. ASyS, antisynthetase syndrome; CK, creatine kinase; DM, dermatomyositis; HCQ, hydroxychloroquine; IVIg, intravenous immunoglobulin; MMF, mycophenolate mofetil; MMT-8, manual muscle testing 8; rese-cel, resecabtagene autoleucel; TAC, tacrolimus; TIS, total improvement score; U/L, units per liter. Cabaletta Bio: Data on File. TIS responses to rese-cel among all DM and ASyS patients show potential for achieving drug-free remission in patients with refractory myositis TIS Major Moderate Minimal None Discontinued all immunomodulators TIS DM ASyS Patient DM-1^ DM-2 ASyS-1 ASyS-2 Latest follow-up 32 weeks 8 weeks 12 weeks 8 weeks TIS Response Major Major Major Minimal to moderate GC-free IM-free DM-1 DM-2 ASyS-1 ASyS-2 MMF, HCQ IVIg, MMF, HCQ IVIg, TAC IVIg, MMF, TAC † ASyS-2 would not meet pivotal entry criteria based on MMT score greater than the planned minimum required for inclusion. Max TIS score possible for this patient is 65.

Lupus: Unmet Need & Clinical Data
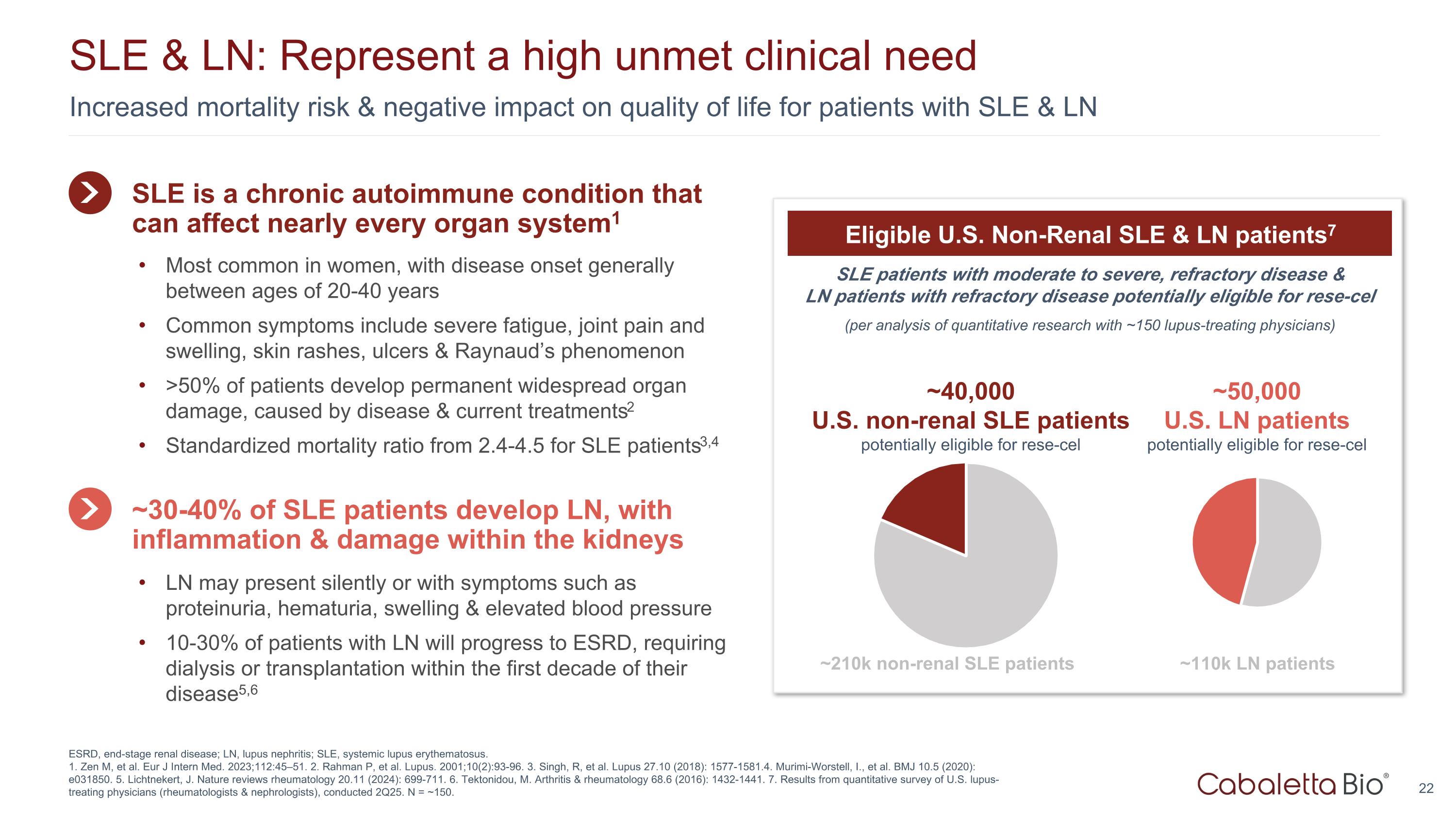
Increased mortality risk & negative impact on quality of life for patients with SLE & LN SLE & LN: Represent a high unmet clinical need ESRD, end-stage renal disease; LN, lupus nephritis; SLE, systemic lupus erythematosus. 1. Zen M, et al. Eur J Intern Med. 2023;112:45–51. 2. Rahman P, et al. Lupus. 2001;10(2):93-96. 3. Singh, R, et al. Lupus 27.10 (2018): 1577-1581.4. Murimi-Worstell, I., et al. BMJ 10.5 (2020): e031850. 5. Lichtnekert, J. Nature reviews rheumatology 20.11 (2024): 699-711. 6. Tektonidou, M. Arthritis & rheumatology 68.6 (2016): 1432-1441. 7. Results from quantitative survey of U.S. lupus-treating physicians (rheumatologists & nephrologists), conducted 2Q25. N = ~150. SLE patients with moderate to severe, refractory disease & LN patients with refractory disease potentially eligible for rese-cel (per analysis of quantitative research with ~150 lupus-treating physicians) Eligible U.S. Non-Renal SLE & LN patients7 ~40,000 U.S. non-renal SLE patients potentially eligible for rese-cel ~210k non-renal SLE patients ~50,000 U.S. LN patients potentially eligible for rese-cel ~110k LN patients SLE is a chronic autoimmune condition that can affect nearly every organ system1 Most common in women, with disease onset generally between ages of 20-40 years Common symptoms include severe fatigue, joint pain and swelling, skin rashes, ulcers & Raynaud’s phenomenon >50% of patients develop permanent widespread organ damage, caused by disease & current treatments2 Standardized mortality ratio from 2.4-4.5 for SLE patients3,4 ~30-40% of SLE patients develop LN, with inflammation & damage within the kidneys LN may present silently or with symptoms such as proteinuria, hematuria, swelling & elevated blood pressure 10-30% of patients with LN will progress to ESRD, requiring dialysis or transplantation within the first decade of their disease5,6
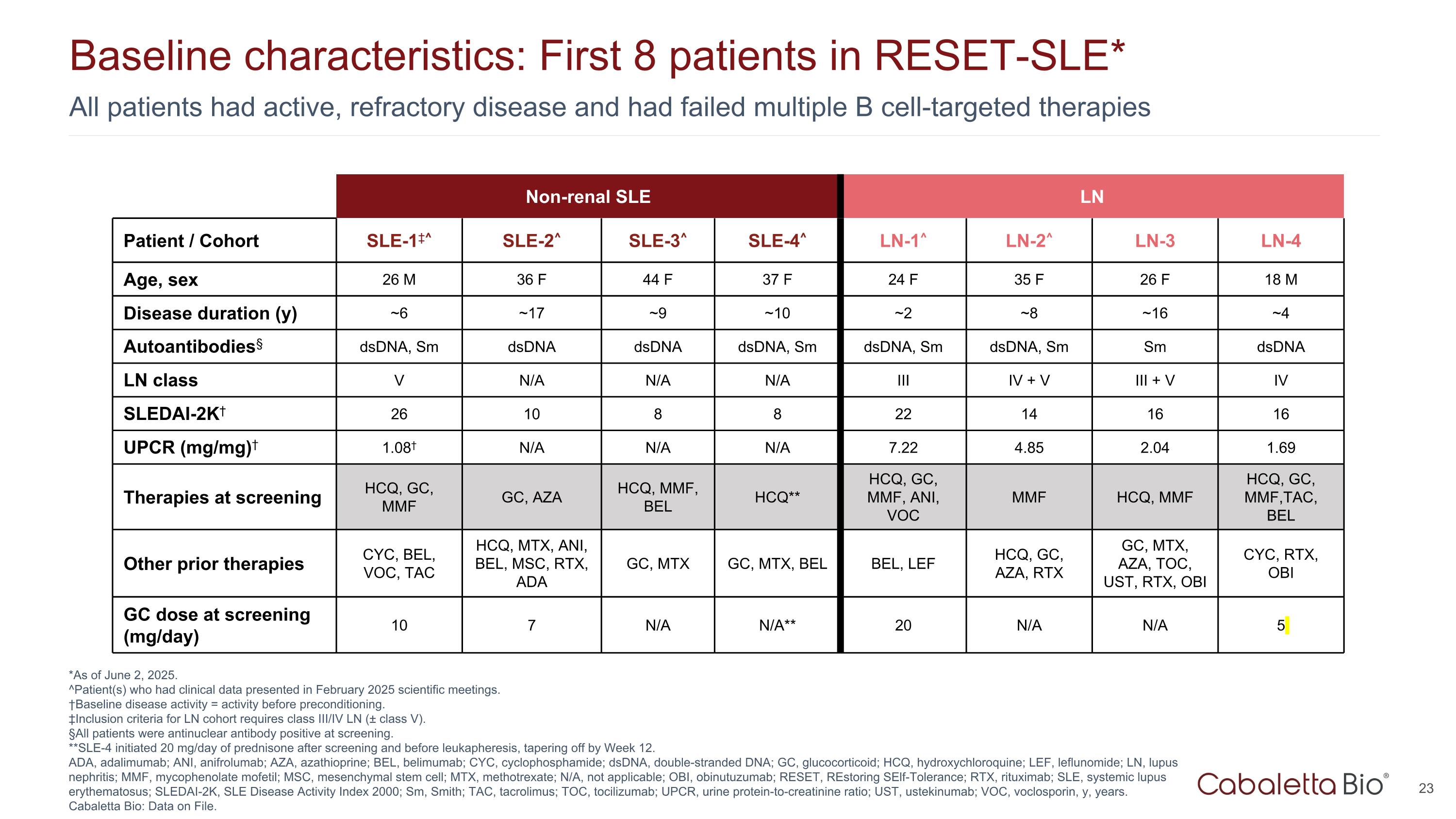
All patients had active, refractory disease and had failed multiple B cell-targeted therapies Baseline characteristics: First 8 patients in RESET-SLE* *As of June 2, 2025. ^Patient(s) who had clinical data presented in February 2025 scientific meetings. †Baseline disease activity = activity before preconditioning. ‡Inclusion criteria for LN cohort requires class III/IV LN (± class V). §All patients were antinuclear antibody positive at screening. **SLE-4 initiated 20 mg/day of prednisone after screening and before leukapheresis, tapering off by Week 12. ADA, adalimumab; ANI, anifrolumab; AZA, azathioprine; BEL, belimumab; CYC, cyclophosphamide; dsDNA, double-stranded DNA; GC, glucocorticoid; HCQ, hydroxychloroquine; LEF, leflunomide; LN, lupus nephritis; MMF, mycophenolate mofetil; MSC, mesenchymal stem cell; MTX, methotrexate; N/A, not applicable; OBI, obinutuzumab; RESET, REstoring SElf-Tolerance; RTX, rituximab; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE Disease Activity Index 2000; Sm, Smith; TAC, tacrolimus; TOC, tocilizumab; UPCR, urine protein-to-creatinine ratio; UST, ustekinumab; VOC, voclosporin, y, years. Cabaletta Bio: Data on File. Non-renal SLE LN Patient / Cohort SLE-1‡^ SLE-2^ SLE-3^ SLE-4^ LN-1^ LN-2^ LN-3 LN-4 Age, sex 26 M 36 F 44 F 37 F 24 F 35 F 26 F 18 M Disease duration (y) ~6 ~17 ~9 ~10 ~2 ~8 ~16 ~4 Autoantibodies§ dsDNA, Sm dsDNA dsDNA dsDNA, Sm dsDNA, Sm dsDNA, Sm Sm dsDNA LN class V N/A N/A N/A III IV + V III + V IV SLEDAI-2K† 26 10 8 8 22 14 16 16 UPCR (mg/mg)† 1.08† N/A N/A N/A 7.22 4.85 2.04 1.69 Therapies at screening HCQ, GC, MMF GC, AZA HCQ, MMF, BEL HCQ** HCQ, GC, MMF, ANI, VOC MMF HCQ, MMF HCQ, GC, MMF,TAC, BEL Other prior therapies CYC, BEL, VOC, TAC HCQ, MTX, ANI, BEL, MSC, RTX, ADA GC, MTX GC, MTX, BEL BEL, LEF HCQ, GC, AZA, RTX GC, MTX, AZA, TOC, UST, RTX, OBI CYC, RTX, OBI GC dose at screening (mg/day) 10 7 N/A N/A** 20 N/A N/A 5
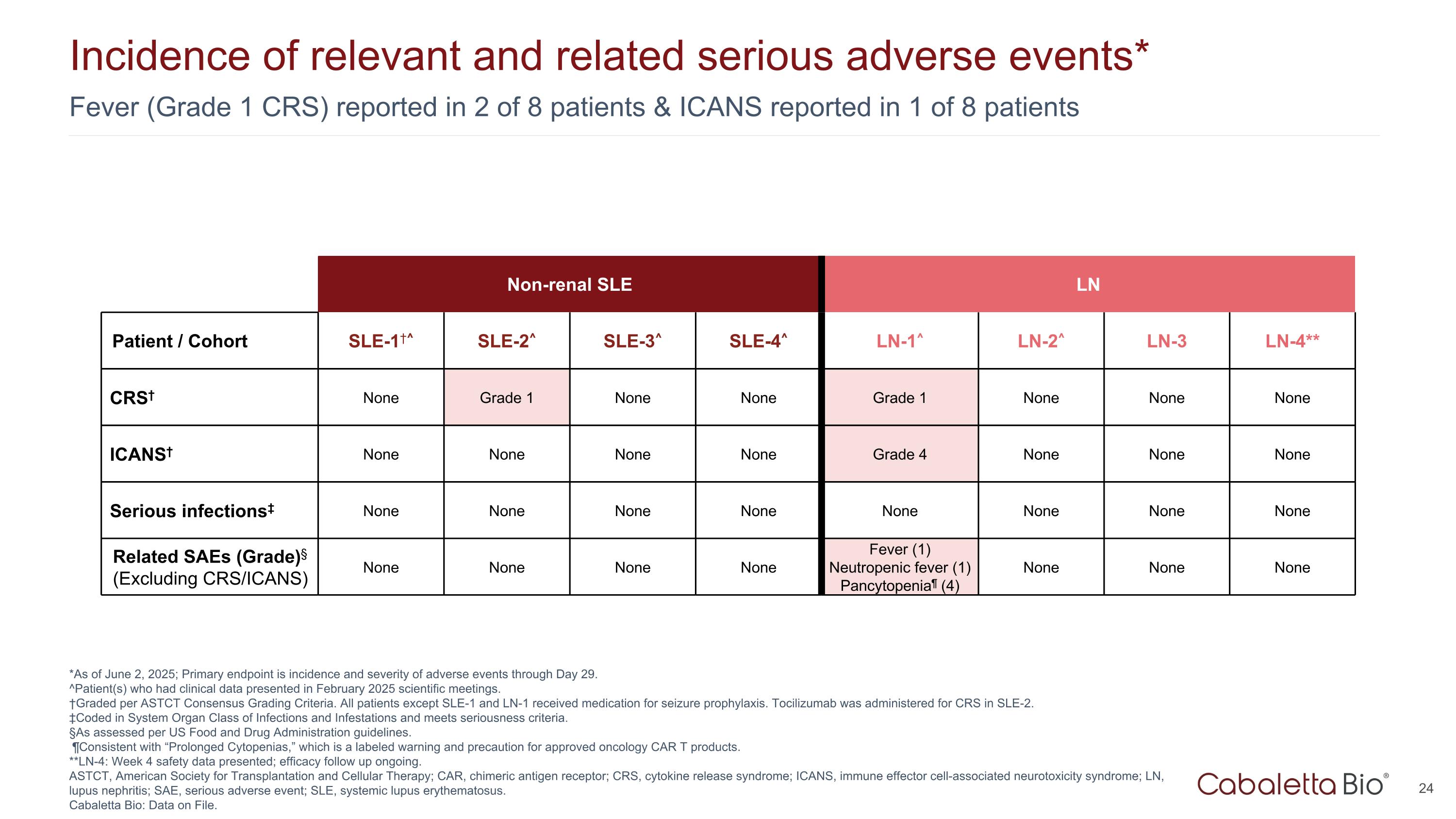
Fever (Grade 1 CRS) reported in 2 of 8 patients & ICANS reported in 1 of 8 patients Incidence of relevant and related serious adverse events* *As of June 2, 2025; Primary endpoint is incidence and severity of adverse events through Day 29. ^Patient(s) who had clinical data presented in February 2025 scientific meetings. †Graded per ASTCT Consensus Grading Criteria. All patients except SLE-1 and LN-1 received medication for seizure prophylaxis. Tocilizumab was administered for CRS in SLE-2. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per US Food and Drug Administration guidelines. ¶Consistent with “Prolonged Cytopenias,” which is a labeled warning and precaution for approved oncology CAR T products. **LN-4: Week 4 safety data presented; efficacy follow up ongoing. ASTCT, American Society for Transplantation and Cellular Therapy; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; LN, lupus nephritis; SAE, serious adverse event; SLE, systemic lupus erythematosus. Cabaletta Bio: Data on File. Non-renal SLE LN Patient / Cohort SLE-1†^ SLE-2^ SLE-3^ SLE-4^ LN-1^ LN-2^ LN-3 LN-4** CRS† None Grade 1 None None Grade 1 None None None ICANS† None None None None Grade 4 None None None Serious infections‡ None None None None None None None None Related SAEs (Grade)§ (Excluding CRS/ICANS) None None None None Fever (1) Neutropenic fever (1) Pancytopenia¶ (4) None None None
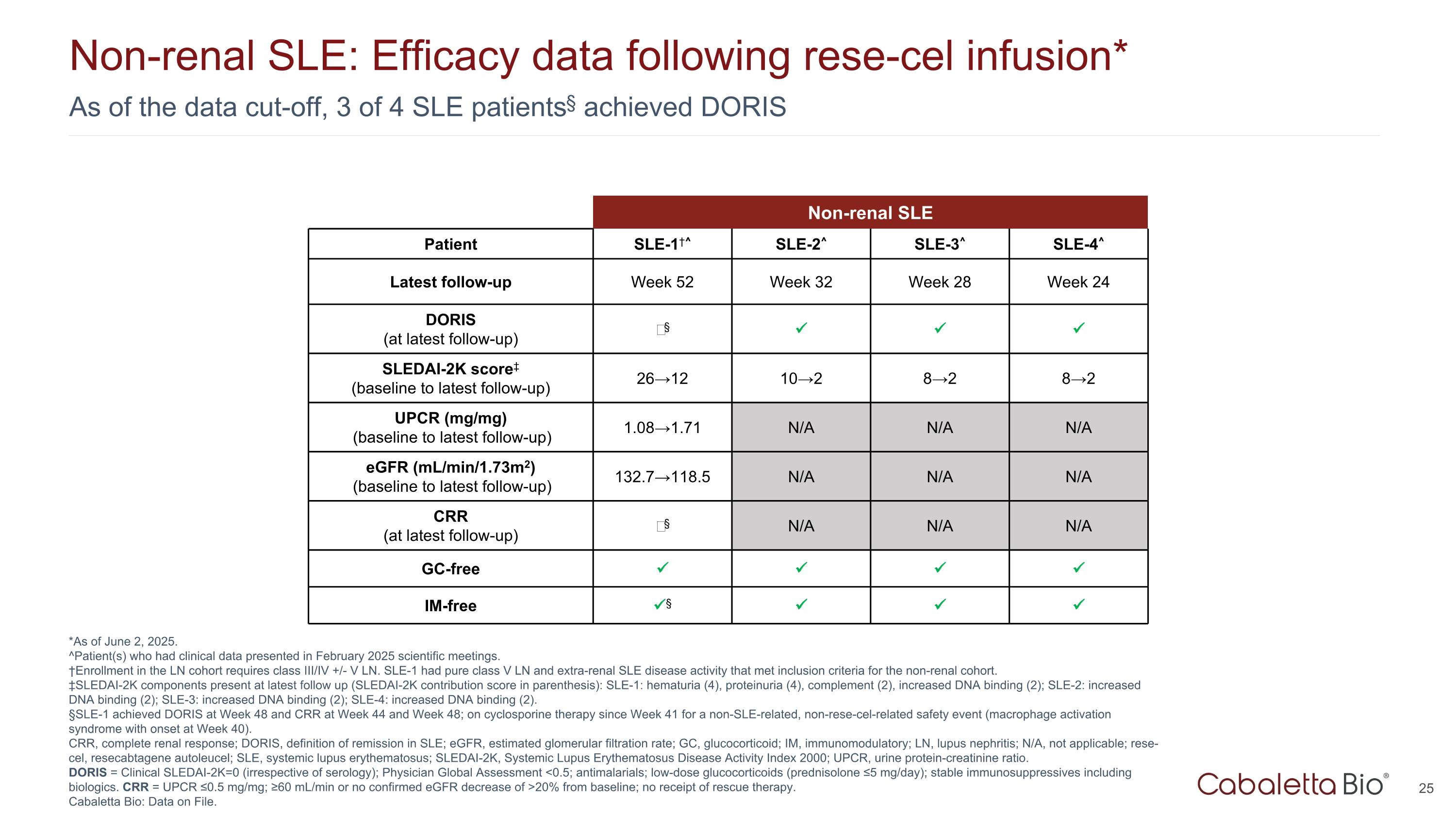
As of the data cut-off, 3 of 4 SLE patients§ achieved DORIS Non-renal SLE: Efficacy data following rese-cel infusion* *As of June 2, 2025. ^Patient(s) who had clinical data presented in February 2025 scientific meetings. †Enrollment in the LN cohort requires class III/IV +/- V LN. SLE-1 had pure class V LN and extra-renal SLE disease activity that met inclusion criteria for the non-renal cohort. ‡SLEDAI-2K components present at latest follow up (SLEDAI-2K contribution score in parenthesis): SLE-1: hematuria (4), proteinuria (4), complement (2), increased DNA binding (2); SLE-2: increased DNA binding (2); SLE-3: increased DNA binding (2); SLE-4: increased DNA binding (2). §SLE-1 achieved DORIS at Week 48 and CRR at Week 44 and Week 48; on cyclosporine therapy since Week 41 for a non-SLE-related, non-rese-cel-related safety event (macrophage activation syndrome with onset at Week 40). CRR, complete renal response; DORIS, definition of remission in SLE; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; IM, immunomodulatory; LN, lupus nephritis; N/A, not applicable; rese-cel, resecabtagene autoleucel; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urine protein-creatinine ratio. DORIS = Clinical SLEDAI-2K=0 (irrespective of serology); Physician Global Assessment <0.5; antimalarials; low-dose glucocorticoids (prednisolone ≤5 mg/day); stable immunosuppressives including biologics. CRR = UPCR ≤0.5 mg/mg; ≥60 mL/min or no confirmed eGFR decrease of >20% from baseline; no receipt of rescue therapy. Cabaletta Bio: Data on File. Non-renal SLE Patient SLE-1†^ SLE-2^ SLE-3^ SLE-4^ Latest follow-up Week 52 Week 32 Week 28 Week 24 DORIS (at latest follow-up) ‒§ SLEDAI-2K score‡ (baseline to latest follow-up) 26→12 10→2 8→2 8→2 UPCR (mg/mg) (baseline to latest follow-up) 1.08→1.71 N/A N/A N/A eGFR (mL/min/1.73m2) (baseline to latest follow-up) 132.7→118.5 N/A N/A N/A CRR (at latest follow-up) ‒§ N/A N/A N/A GC-free IM-free §
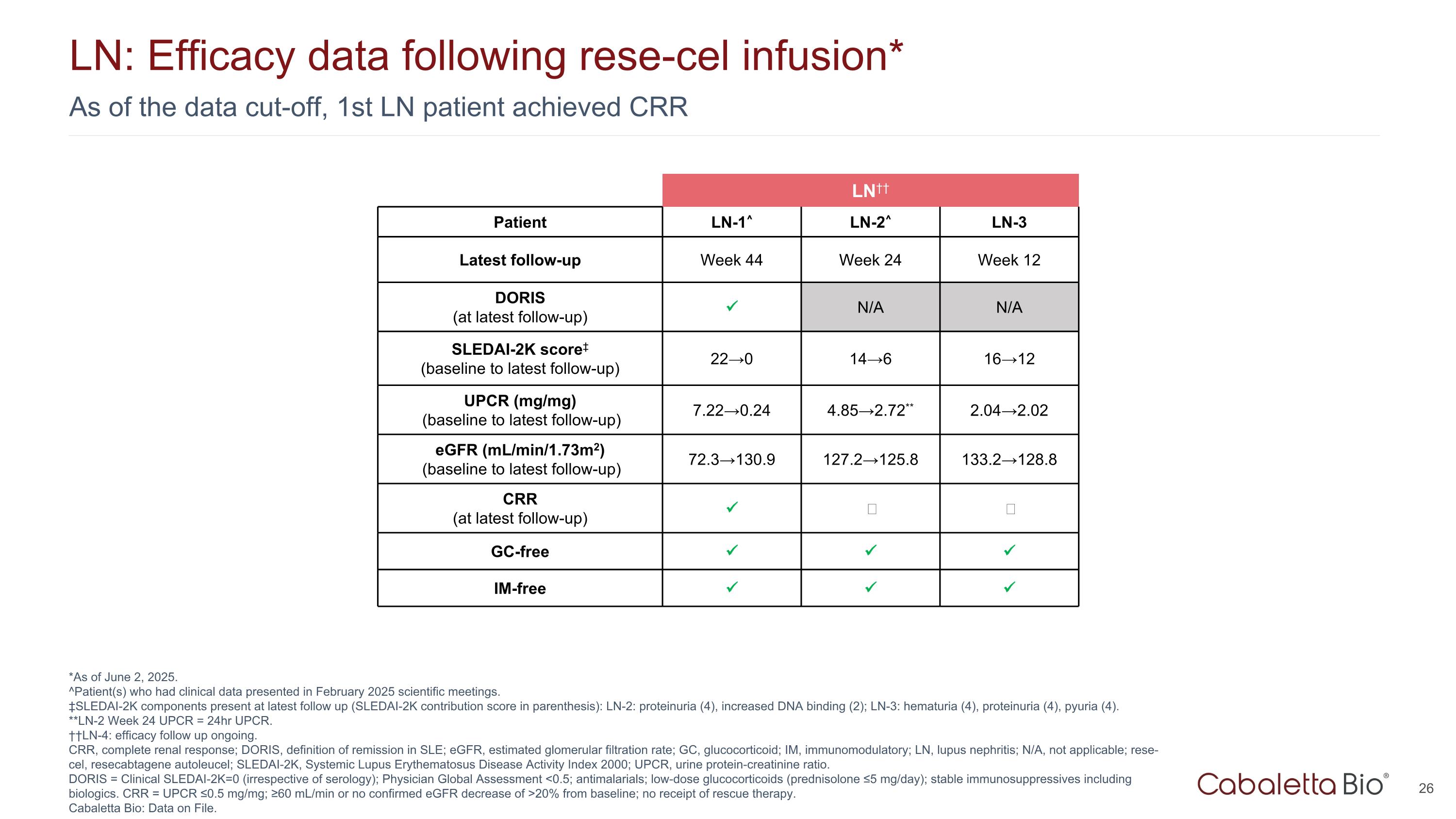
As of the data cut-off, 1st LN patient achieved CRR LN: Efficacy data following rese-cel infusion* *As of June 2, 2025. ^Patient(s) who had clinical data presented in February 2025 scientific meetings. ‡SLEDAI-2K components present at latest follow up (SLEDAI-2K contribution score in parenthesis): LN-2: proteinuria (4), increased DNA binding (2); LN-3: hematuria (4), proteinuria (4), pyuria (4). **LN-2 Week 24 UPCR = 24hr UPCR. ††LN-4: efficacy follow up ongoing. CRR, complete renal response; DORIS, definition of remission in SLE; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; IM, immunomodulatory; LN, lupus nephritis; N/A, not applicable; rese-cel, resecabtagene autoleucel; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000; UPCR, urine protein-creatinine ratio. DORIS = Clinical SLEDAI-2K=0 (irrespective of serology); Physician Global Assessment <0.5; antimalarials; low-dose glucocorticoids (prednisolone ≤5 mg/day); stable immunosuppressives including biologics. CRR = UPCR ≤0.5 mg/mg; ≥60 mL/min or no confirmed eGFR decrease of >20% from baseline; no receipt of rescue therapy. Cabaletta Bio: Data on File. LN†† Patient LN-1^ LN-2^ LN-3 Latest follow-up Week 44 Week 24 Week 12 DORIS (at latest follow-up) N/A N/A SLEDAI-2K score‡ (baseline to latest follow-up) 22→0 14→6 16→12 UPCR (mg/mg) (baseline to latest follow-up) 7.22→0.24 4.85→2.72** 2.04→2.02 eGFR (mL/min/1.73m2) (baseline to latest follow-up) 72.3→130.9 127.2→125.8 133.2→128.8 CRR (at latest follow-up) ‒ ‒ GC-free IM-free

Systemic Sclerosis: Unmet Need & Clinical Data
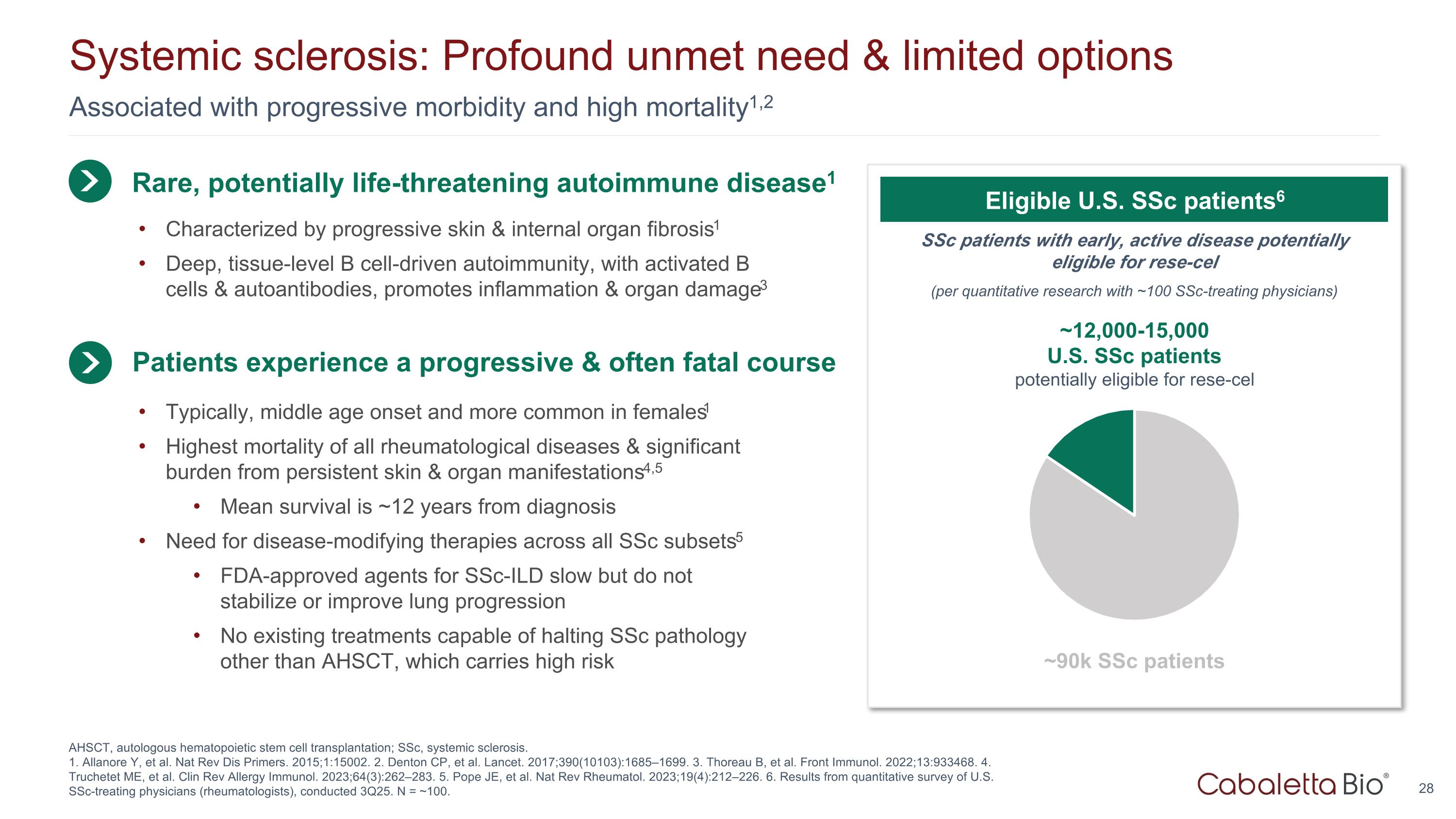
Associated with progressive morbidity and high mortality1,2 Systemic sclerosis: Profound unmet need & limited options AHSCT, autologous hematopoietic stem cell transplantation; SSc, systemic sclerosis. 1. Allanore Y, et al. Nat Rev Dis Primers. 2015;1:15002. 2. Denton CP, et al. Lancet. 2017;390(10103):1685–1699. 3. Thoreau B, et al. Front Immunol. 2022;13:933468. 4. Truchetet ME, et al. Clin Rev Allergy Immunol. 2023;64(3):262–283. 5. Pope JE, et al. Nat Rev Rheumatol. 2023;19(4):212–226. 6. Results from quantitative survey of U.S. SSc-treating physicians (rheumatologists), conducted 3Q25. N = ~100. Rare, potentially life-threatening autoimmune disease1 Typically, middle age onset and more common in females1 Highest mortality of all rheumatological diseases & significant burden from persistent skin & organ manifestations4,5 Mean survival is ~12 years from diagnosis Need for disease-modifying therapies across all SSc subsets5 FDA-approved agents for SSc-ILD slow but do not stabilize or improve lung progression No existing treatments capable of halting SSc pathology other than AHSCT, which carries high risk Patients experience a progressive & often fatal course SSc patients with early, active disease potentially eligible for rese-cel (per quantitative research with ~100 SSc-treating physicians) Eligible U.S. SSc patients6 ~12,000-15,000 U.S. SSc patients potentially eligible for rese-cel ~90k SSc patients Characterized by progressive skin & internal organ fibrosis1 Deep, tissue-level B cell-driven autoimmunity, with activated B cells & autoantibodies, promotes inflammation & organ damage3
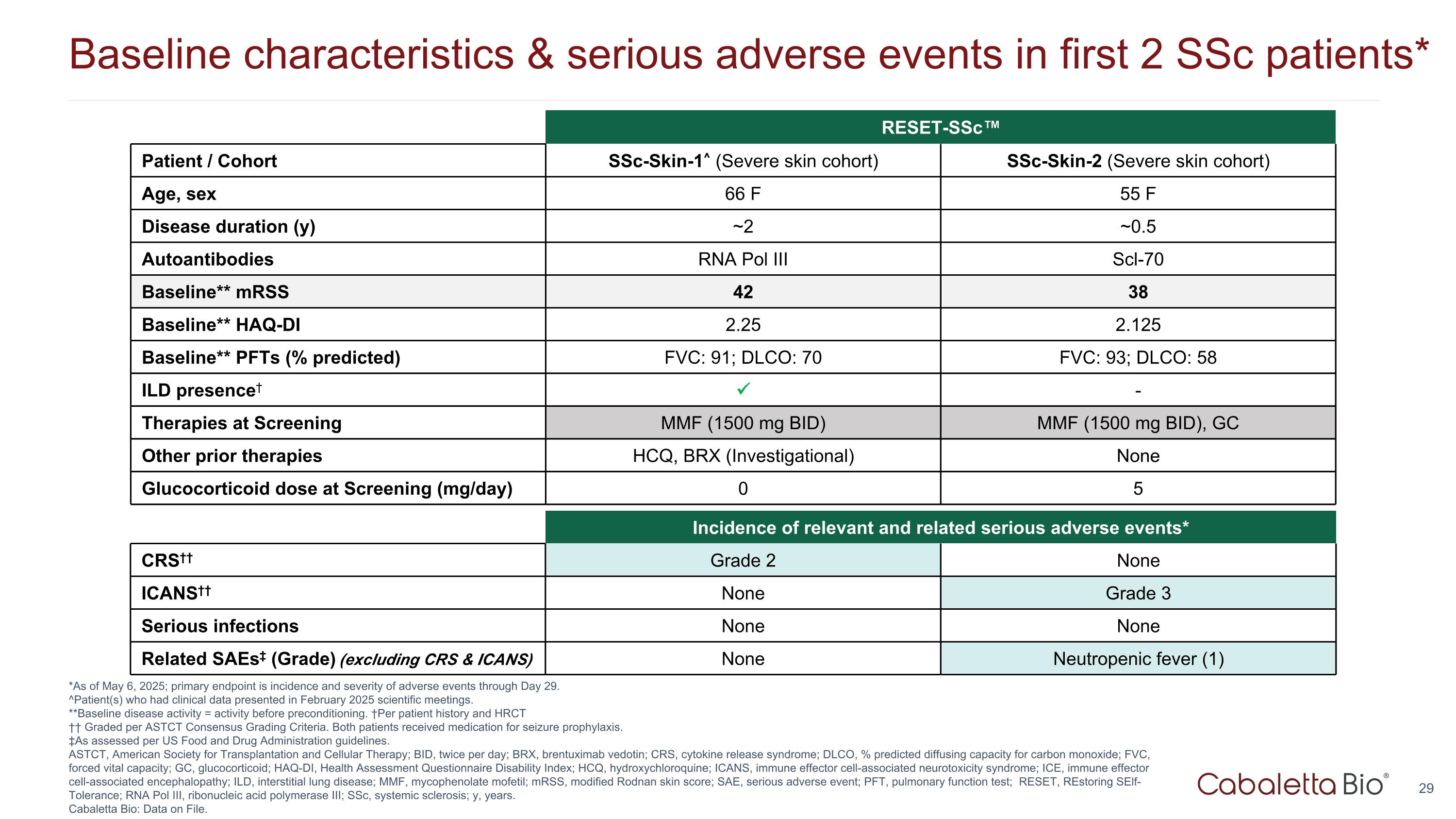
Baseline characteristics & serious adverse events in first 2 SSc patients* *As of May 6, 2025; primary endpoint is incidence and severity of adverse events through Day 29. ^Patient(s) who had clinical data presented in February 2025 scientific meetings. **Baseline disease activity = activity before preconditioning. †Per patient history and HRCT †† Graded per ASTCT Consensus Grading Criteria. Both patients received medication for seizure prophylaxis. ‡As assessed per US Food and Drug Administration guidelines. ASTCT, American Society for Transplantation and Cellular Therapy; BID, twice per day; BRX, brentuximab vedotin; CRS, cytokine release syndrome; DLCO, % predicted diffusing capacity for carbon monoxide; FVC, forced vital capacity; GC, glucocorticoid; HAQ-DI, Health Assessment Questionnaire Disability Index; HCQ, hydroxychloroquine; ICANS, immune effector cell-associated neurotoxicity syndrome; ICE, immune effector cell-associated encephalopathy; ILD, interstitial lung disease; MMF, mycophenolate mofetil; mRSS, modified Rodnan skin score; SAE, serious adverse event; PFT, pulmonary function test; RESET, REstoring SElf-Tolerance; RNA Pol III, ribonucleic acid polymerase III; SSc, systemic sclerosis; y, years. Cabaletta Bio: Data on File. Incidence of relevant and related serious adverse events* CRS†† Grade 2 None ICANS†† None Grade 3 Serious infections None None Related SAEs‡ (Grade) (excluding CRS & ICANS) None Neutropenic fever (1) RESET-SSc™ Patient / Cohort SSc-Skin-1^ (Severe skin cohort) SSc-Skin-2 (Severe skin cohort) Age, sex 66 F 55 F Disease duration (y) ~2 ~0.5 Autoantibodies RNA Pol III Scl-70 Baseline** mRSS 42 38 Baseline** HAQ-DI 2.25 2.125 Baseline** PFTs (% predicted) FVC: 91; DLCO: 70 FVC: 93; DLCO: 58 ILD presence† - Therapies at Screening MMF (1500 mg BID) MMF (1500 mg BID), GC Other prior therapies HCQ, BRX (Investigational) None Glucocorticoid dose at Screening (mg/day) 0 5
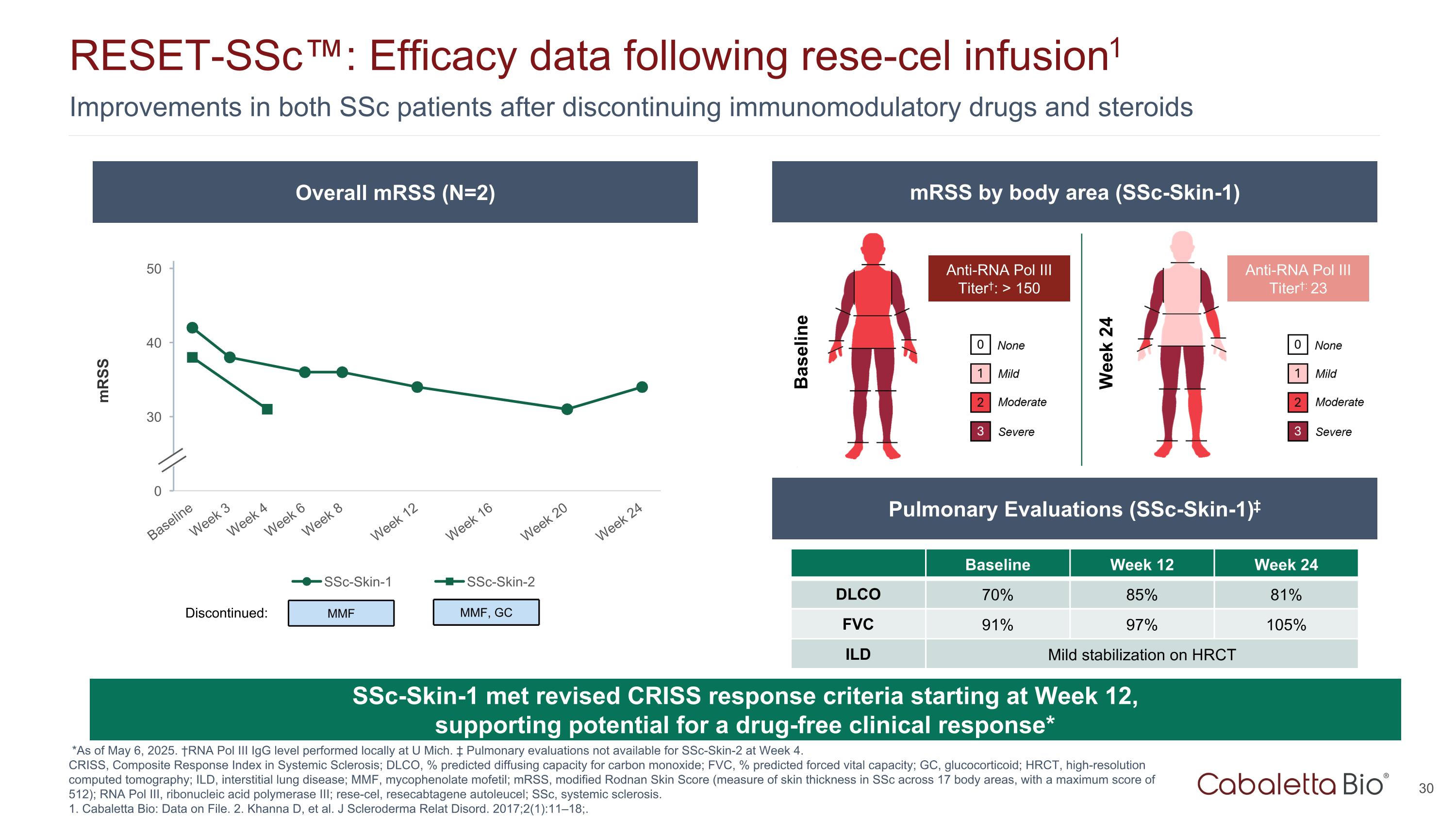
Improvements in both SSc patients after discontinuing immunomodulatory drugs and steroids RESET-SSc™: Efficacy data following rese-cel infusion1 *As of May 6, 2025. †RNA Pol III IgG level performed locally at U Mich. ‡ Pulmonary evaluations not available for SSc-Skin-2 at Week 4. CRISS, Composite Response Index in Systemic Sclerosis; DLCO, % predicted diffusing capacity for carbon monoxide; FVC, % predicted forced vital capacity; GC, glucocorticoid; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; MMF, mycophenolate mofetil; mRSS, modified Rodnan Skin Score (measure of skin thickness in SSc across 17 body areas, with a maximum score of 512); RNA Pol III, ribonucleic acid polymerase III; rese-cel, resecabtagene autoleucel; SSc, systemic sclerosis. 1. Cabaletta Bio: Data on File. 2. Khanna D, et al. J Scleroderma Relat Disord. 2017;2(1):11–18;. SSc-Skin-1 met revised CRISS response criteria starting at Week 12, supporting potential for a drug-free clinical response* Overall mRSS (N=2) mRSS by body area (SSc-Skin-1) Pulmonary Evaluations (SSc-Skin-1)‡ Baseline Week 24 Anti-RNA Pol III Titer†: > 150 Anti-RNA Pol III Titer†: 23 Baseline Week 12 Week 24 DLCO 70% 85% 81% FVC 91% 97% 105% ILD Mild stabilization on HRCT MMF Discontinued: MMF, GC
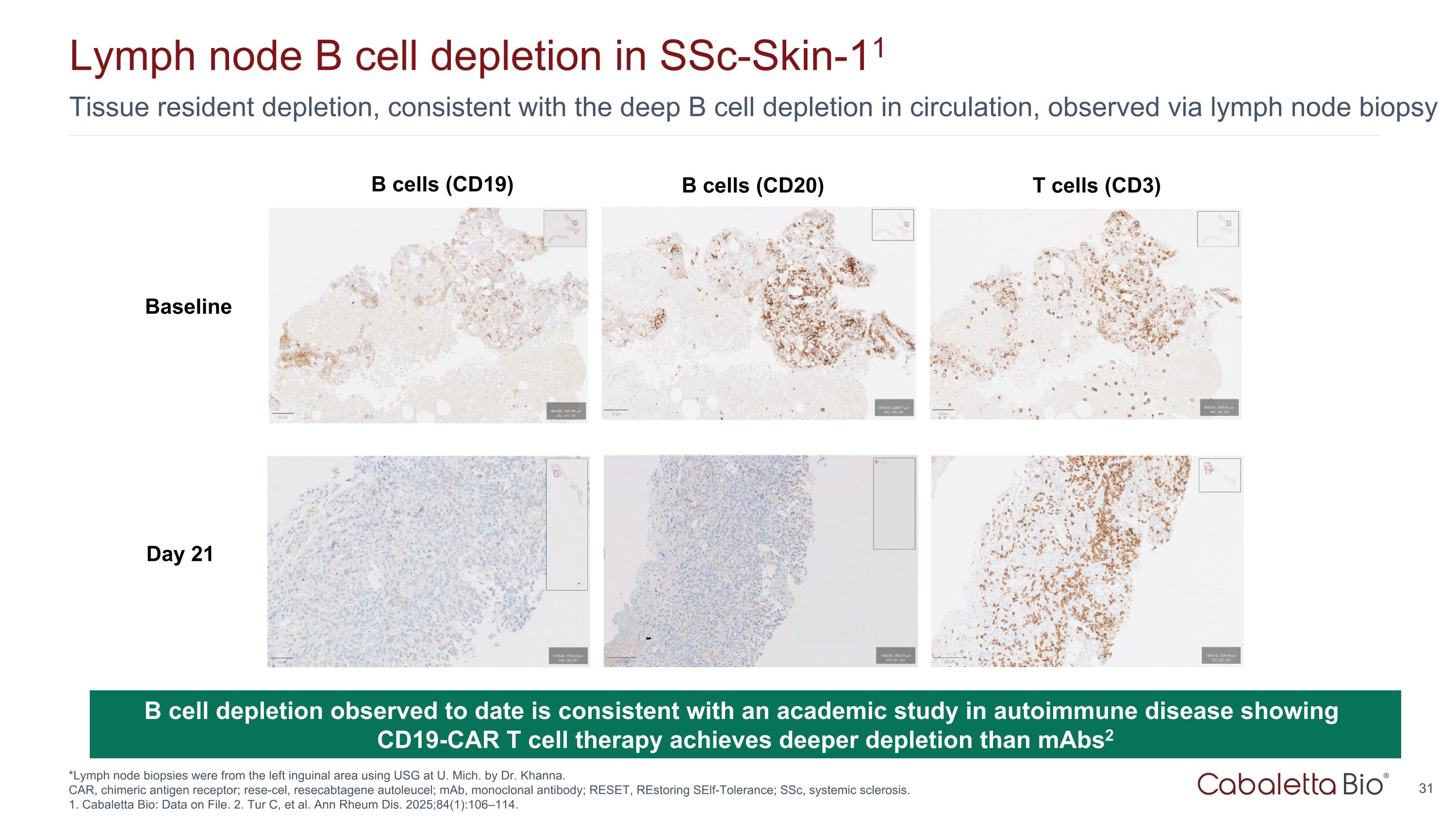
Tissue resident depletion, consistent with the deep B cell depletion in circulation, observed via lymph node biopsy Lymph node B cell depletion in SSc-Skin-11 *Lymph node biopsies were from the left inguinal area using USG at U. Mich. by Dr. Khanna. CAR, chimeric antigen receptor; rese-cel, resecabtagene autoleucel; mAb, monoclonal antibody; RESET, REstoring SElf-Tolerance; SSc, systemic sclerosis. 1. Cabaletta Bio: Data on File. 2. Tur C, et al. Ann Rheum Dis. 2025;84(1):106–114. B cell depletion observed to date is consistent with an academic study in autoimmune disease showing CD19-CAR T cell therapy achieves deeper depletion than mAbs2 Baseline Day 21 T cells (CD3) B cells (CD20) B cells (CD19)

Myasthenia Gravis: Unmet Need
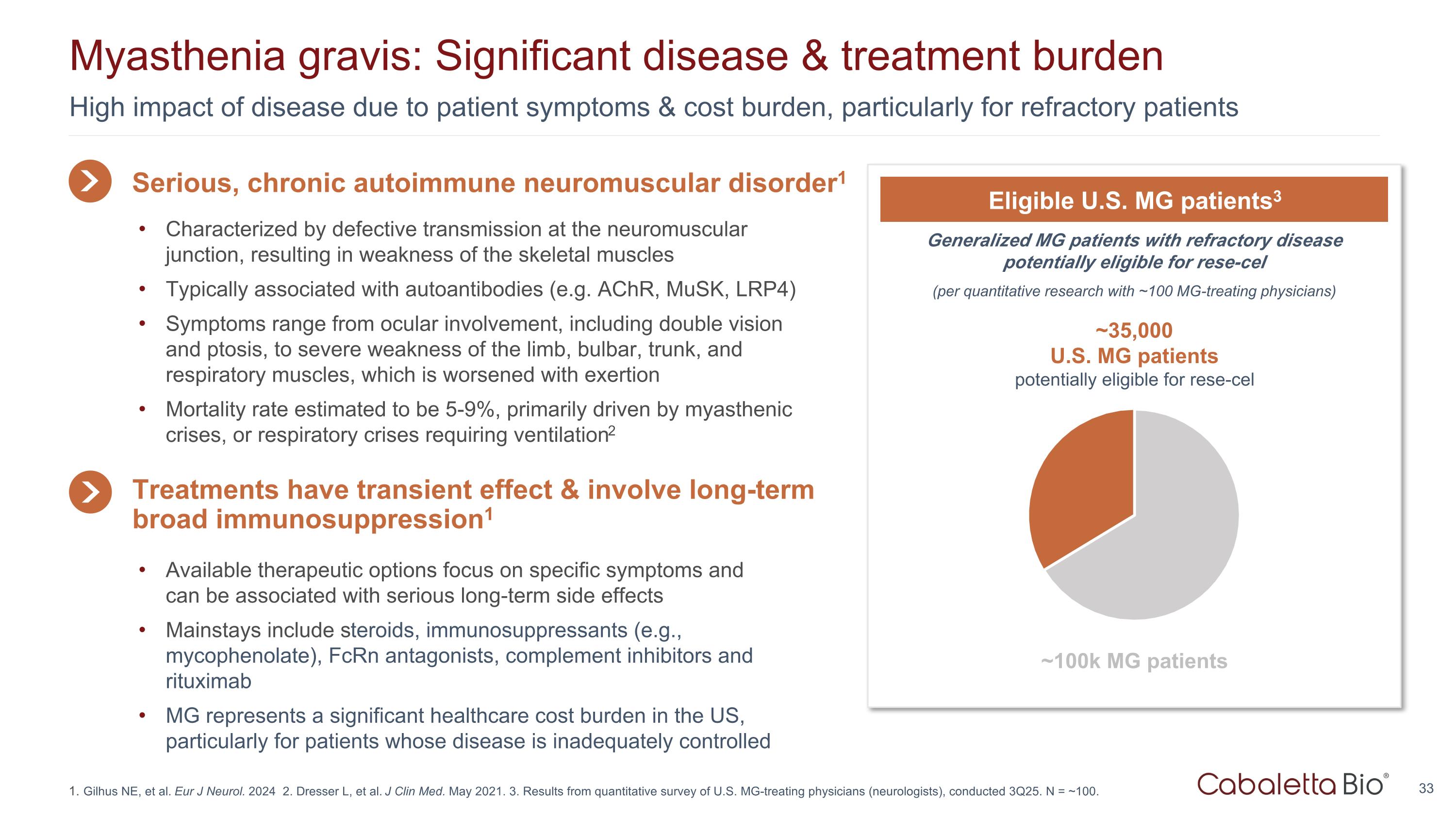
High impact of disease due to patient symptoms & cost burden, particularly for refractory patients Myasthenia gravis: Significant disease & treatment burden Gilhus NE, et al. Eur J Neurol. 2024 2. Dresser L, et al. J Clin Med. May 2021. 3. Results from quantitative survey of U.S. MG-treating physicians (neurologists), conducted 3Q25. N = ~100. Generalized MG patients with refractory disease potentially eligible for rese-cel (per quantitative research with ~100 MG-treating physicians) Eligible U.S. MG patients3 ~35,000 U.S. MG patients potentially eligible for rese-cel ~100k MG patients Serious, chronic autoimmune neuromuscular disorder1 Available therapeutic options focus on specific symptoms and can be associated with serious long-term side effects Mainstays include steroids, immunosuppressants (e.g., mycophenolate), FcRn antagonists, complement inhibitors and rituximab MG represents a significant healthcare cost burden in the US, particularly for patients whose disease is inadequately controlled Treatments have transient effect & involve long-term broad immunosuppression1 Characterized by defective transmission at the neuromuscular junction, resulting in weakness of the skeletal muscles Typically associated with autoantibodies (e.g. AChR, MuSK, LRP4) Symptoms range from ocular involvement, including double vision and ptosis, to severe weakness of the limb, bulbar, trunk, and respiratory muscles, which is worsened with exertion Mortality rate estimated to be 5-9%, primarily driven by myasthenic crises, or respiratory crises requiring ventilation2

Rese-cel – Initial Dose Data without Preconditioning
Clear evidence of biologic and clinical activity in all three PV patients in the initial dose cohort PDAI improvements were present in all three and were compelling in two of the three patients All patients remain off all immunomodulators while GCs are being tapered from low doses Complete B cell depletion was observed in the two patients with the greatest clinical response BAFF induction in these two patients was within the range of rese-cel with PC Rese-cel persistence without PC was similar to patients who received rese-cel with PC Peak persistence was not impacted by absence of PC and occurred slightly later without PC IFNγ induction in non-PC patients was at the higher end of the range observed in PC patients Higher levels may be attributable to higher B cell burden in PV patients and/or absence of preconditioning Rese-cel was generally well tolerated in PV patients without PC1 Based on limited data in the first three patients without PC, CRS rate was similar in rese-cel patients with PC Summary of early clinical and translational data Rese-cel without preconditioning (PC), initial dose cohort* *As of 11 September 2025. Cabaletta Bio: Data on file. BAFF, B cell activating factor; CRS, cytokine release syndrome; GC: glucocorticoids; PDAI, pemphigus disease area index; PV, pemphigus vulgaris; rese-cel, resacabtagene autoleucel; IFNγ, interferon-gamma Standard preconditioning in RESET trials consists of fludarabine 25 mg/m2 x 3 days and cyclophosphamide 1000 mg/m2 x 1 day.

Designed to evaluate the safety and tolerability of rese-cel in PV subjects with active, refractory disease RESET-PV™ phase 1/2 trial: key inclusion & exclusion criteria1 As of 11 September 2025. DSG3, desmoglein 3; PV, pemphigus vulgaris; RESET™, REstoring SElf-Tolerance. Cabaletta Bio: Data on file Age > 18 Confirmed diagnosis of PV by prior or screening biopsy and prior DSG3 antibody positive (reconfirmed during screening) PV inadequately managed by at least one standard immunomodulatory therapy Active PV at screening Key inclusion criteria Key exclusion criteria Have paraneoplastic pemphigus or active malignancy (not including non-melanoma skin cancer) Have received rituximab or other anti-CD20 or anti-CD19 therapies in last 12 months unless anti-DSG3 antibody titers have recently increased or PV symptoms have recently worsened Prednisone > 0.25mg/kg/day Other autoimmune disorder requiring immunomodulatory therapies
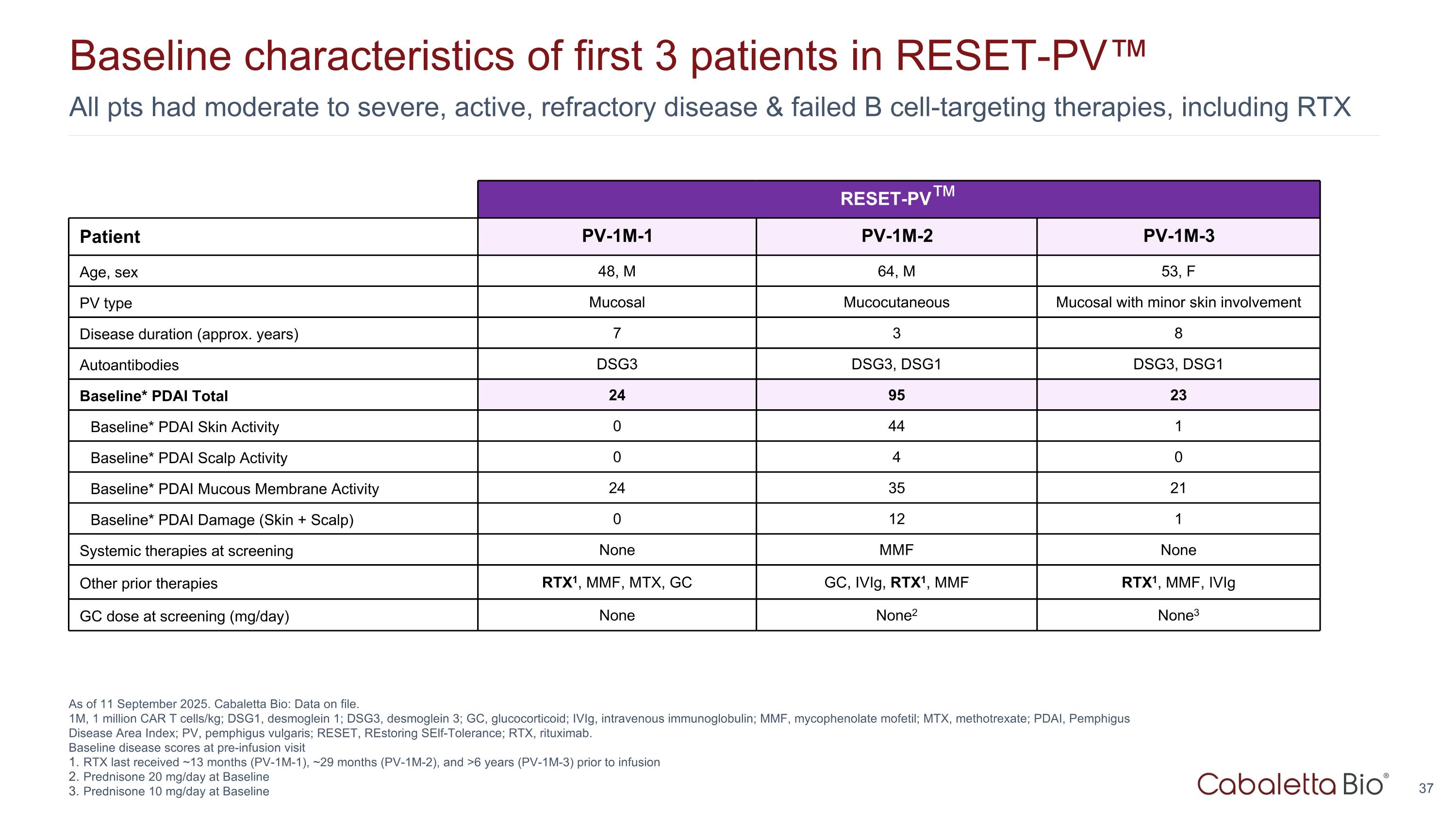
All pts had moderate to severe, active, refractory disease & failed B cell-targeting therapies, including RTX Baseline characteristics of first 3 patients in RESET-PV™ As of 11 September 2025. Cabaletta Bio: Data on file. 1M, 1 million CAR T cells/kg; DSG1, desmoglein 1; DSG3, desmoglein 3; GC, glucocorticoid; IVIg, intravenous immunoglobulin; MMF, mycophenolate mofetil; MTX, methotrexate; PDAI, Pemphigus Disease Area Index; PV, pemphigus vulgaris; RESET, REstoring SElf-Tolerance; RTX, rituximab. Baseline disease scores at pre-infusion visit RTX last received ~13 months (PV-1M-1), ~29 months (PV-1M-2), and >6 years (PV-1M-3) prior to infusion Prednisone 20 mg/day at Baseline Prednisone 10 mg/day at Baseline RESET-PV™ Patient PV-1M-1 PV-1M-2 PV-1M-3 Age, sex 48, M 64, M 53, F PV type Mucosal Mucocutaneous Mucosal with minor skin involvement Disease duration (approx. years) 7 3 8 Autoantibodies DSG3 DSG3, DSG1 DSG3, DSG1 Baseline* PDAI Total 24 95 23 Baseline* PDAI Skin Activity 0 44 1 Baseline* PDAI Scalp Activity 0 4 0 Baseline* PDAI Mucous Membrane Activity 24 35 21 Baseline* PDAI Damage (Skin + Scalp) 0 12 1 Systemic therapies at screening None MMF None Other prior therapies RTX1, MMF, MTX, GC GC, IVIg, RTX1, MMF RTX1, MMF, IVIg GC dose at screening (mg/day) None None2 None3
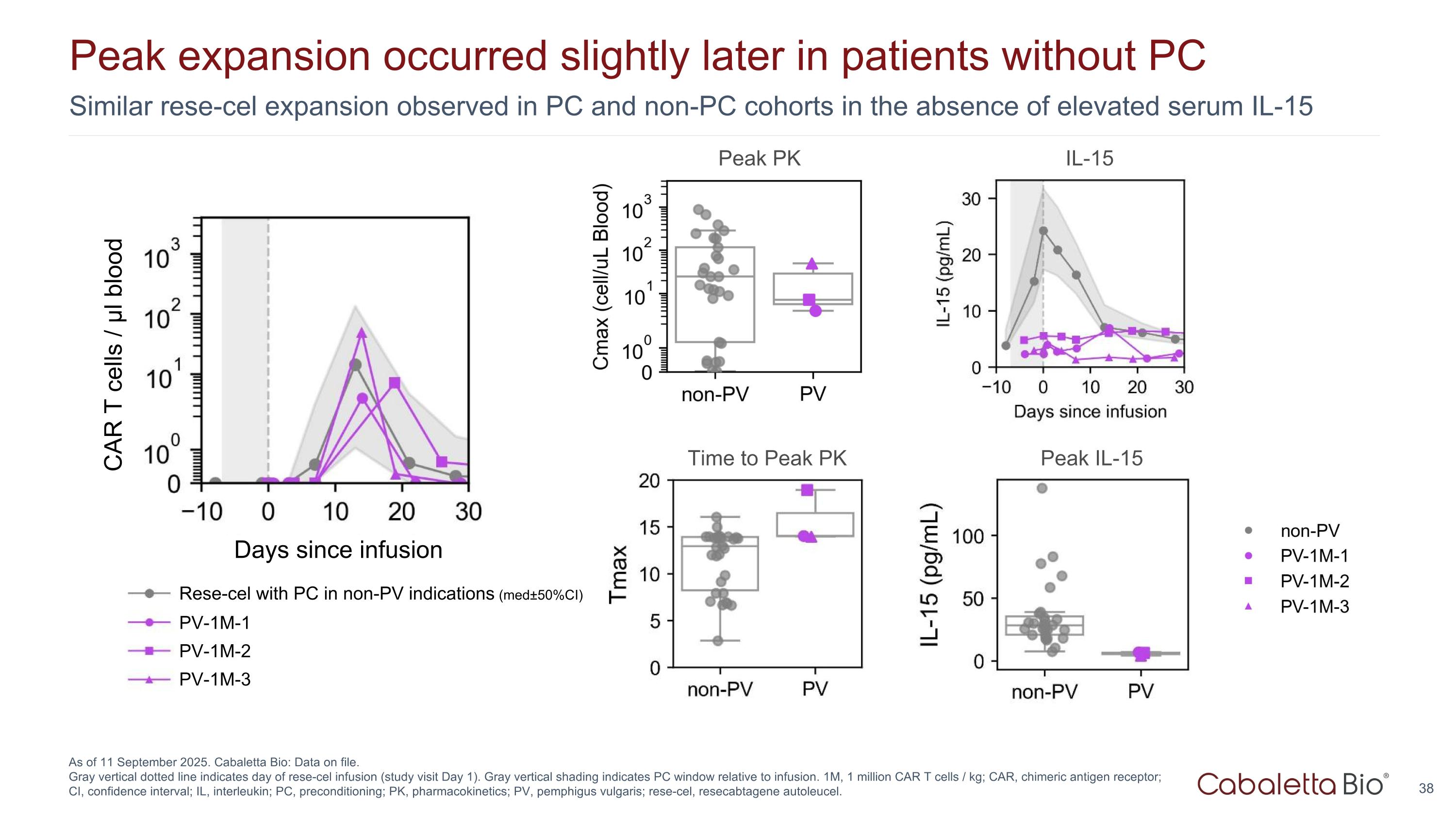
Similar rese-cel expansion observed in PC and non-PC cohorts in the absence of elevated serum IL-15 Peak expansion occurred slightly later in patients without PC As of 11 September 2025. Cabaletta Bio: Data on file. Gray vertical dotted line indicates day of rese-cel infusion (study visit Day 1). Gray vertical shading indicates PC window relative to infusion. 1M, 1 million CAR T cells / kg; CAR, chimeric antigen receptor; CI, confidence interval; IL, interleukin; PC, preconditioning; PK, pharmacokinetics; PV, pemphigus vulgaris; rese-cel, resecabtagene autoleucel. Days since infusion PV-1M-1 PV-1M-2 PV-1M-3 Rese-cel with PC in non-PV indications (med±50%CI) IL-15 Peak PK Peak IL-15 Time to Peak PK CAR T cells / μl blood
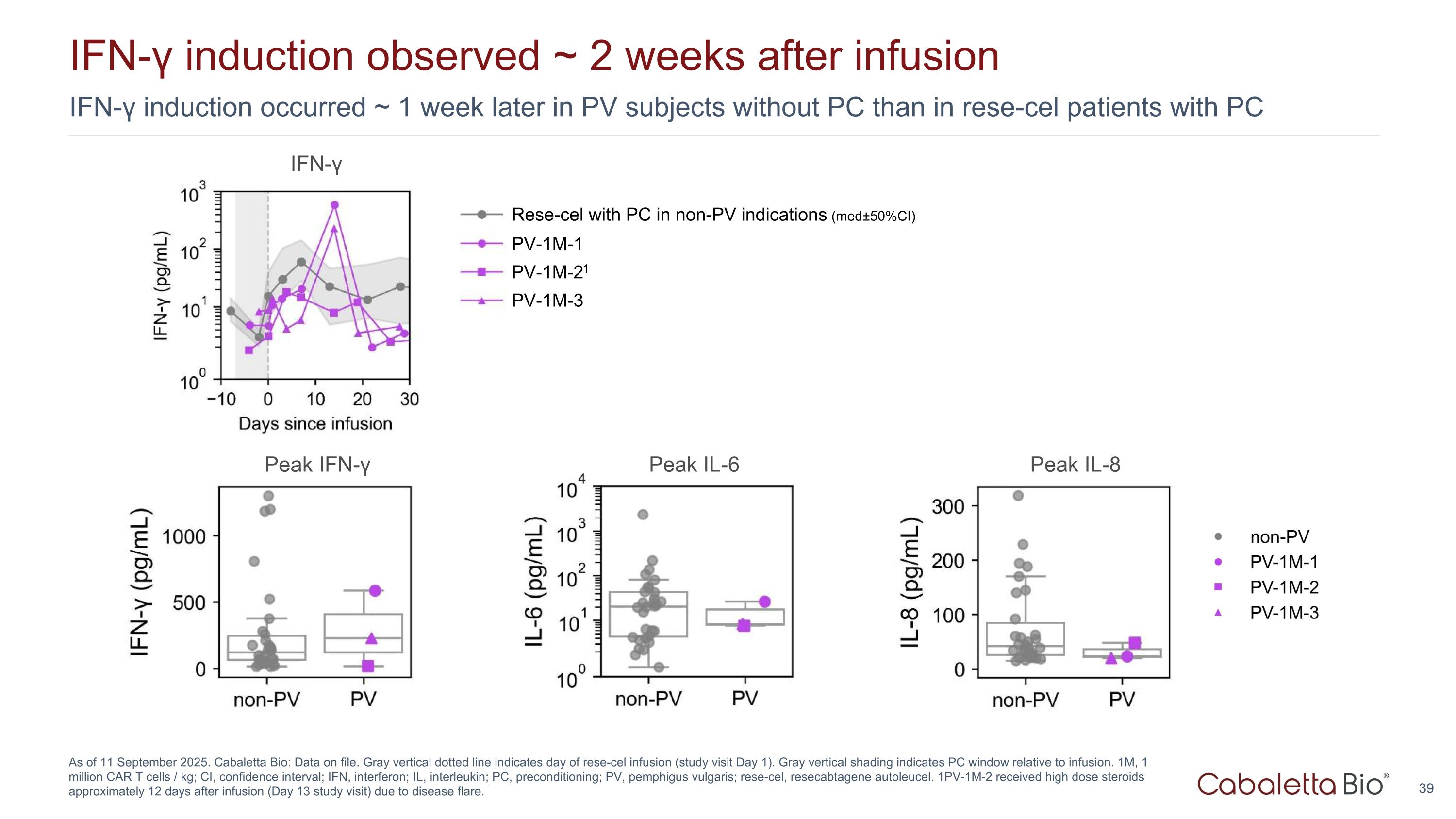
IFN-γ induction occurred ~ 1 week later in PV subjects without PC than in rese-cel patients with PC IFN-γ induction observed ~ 2 weeks after infusion As of 11 September 2025. Cabaletta Bio: Data on file. Gray vertical dotted line indicates day of rese-cel infusion (study visit Day 1). Gray vertical shading indicates PC window relative to infusion. 1M, 1 million CAR T cells / kg; CI, confidence interval; IFN, interferon; IL, interleukin; PC, preconditioning; PV, pemphigus vulgaris; rese-cel, resecabtagene autoleucel. 1PV-1M-2 received high dose steroids approximately 12 days after infusion (Day 13 study visit) due to disease flare. Peak IFN-γ Peak IL-6 Peak IL-8 IFN-γ PV-1M-1 PV-1M-21 PV-1M-3 Rese-cel with PC in non-PV indications (med±50%CI)
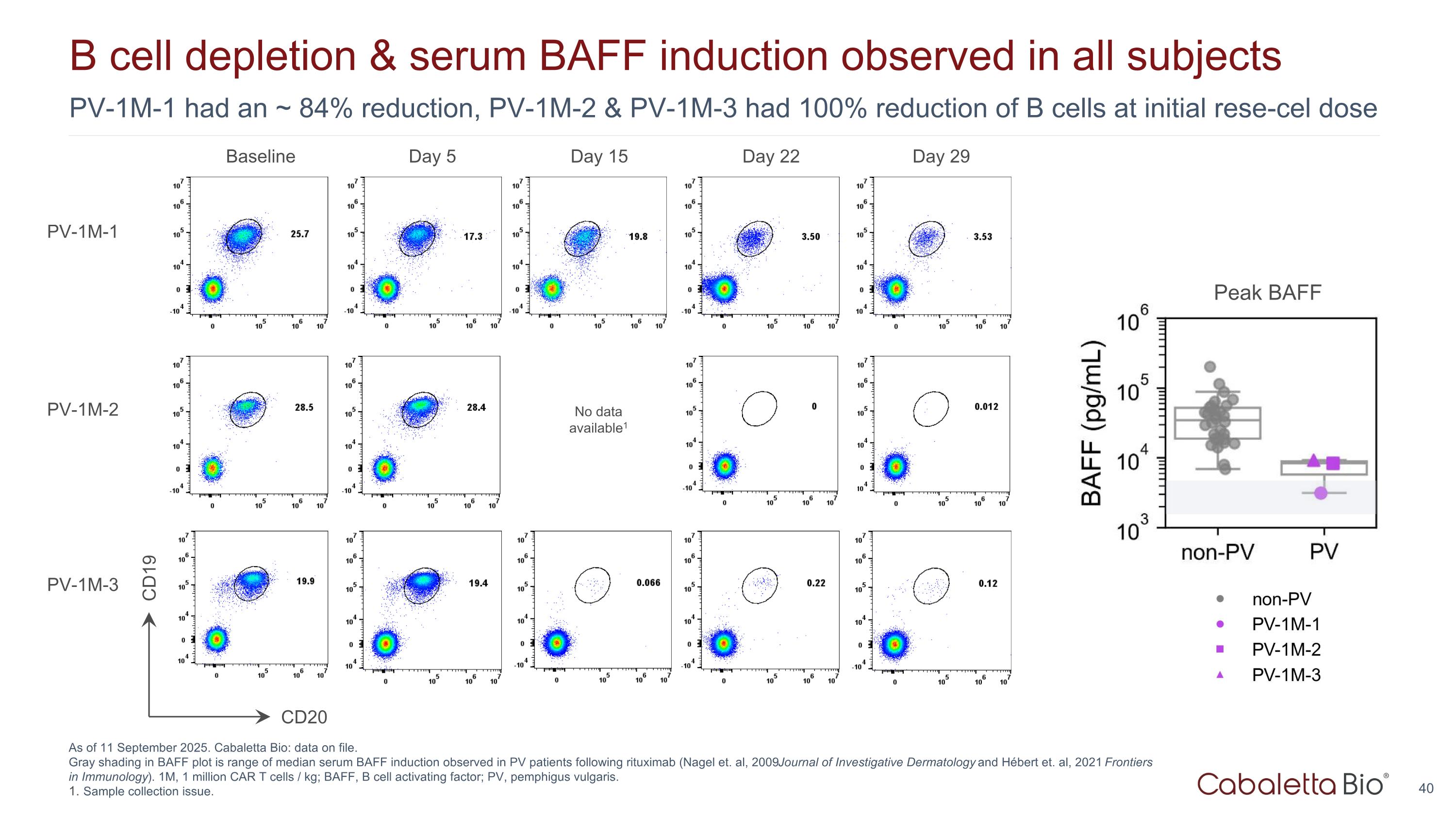
PV-1M-1 had an ~ 84% reduction, PV-1M-2 & PV-1M-3 had 100% reduction of B cells at initial rese-cel dose B cell depletion & serum BAFF induction observed in all subjects As of 11 September 2025. Cabaletta Bio: data on file. Gray shading in BAFF plot is range of median serum BAFF induction observed in PV patients following rituximab (Nagel et. al, 2009 Journal of Investigative Dermatology and Hébert et. al, 2021 Frontiers in Immunology). 1M, 1 million CAR T cells / kg; BAFF, B cell activating factor; PV, pemphigus vulgaris. Sample collection issue. Baseline Day 5 Day 15 Day 22 Day 29 CD20 CD19 PV-1M-1 PV-1M-2 PV-1M-3 No data available1 Peak BAFF
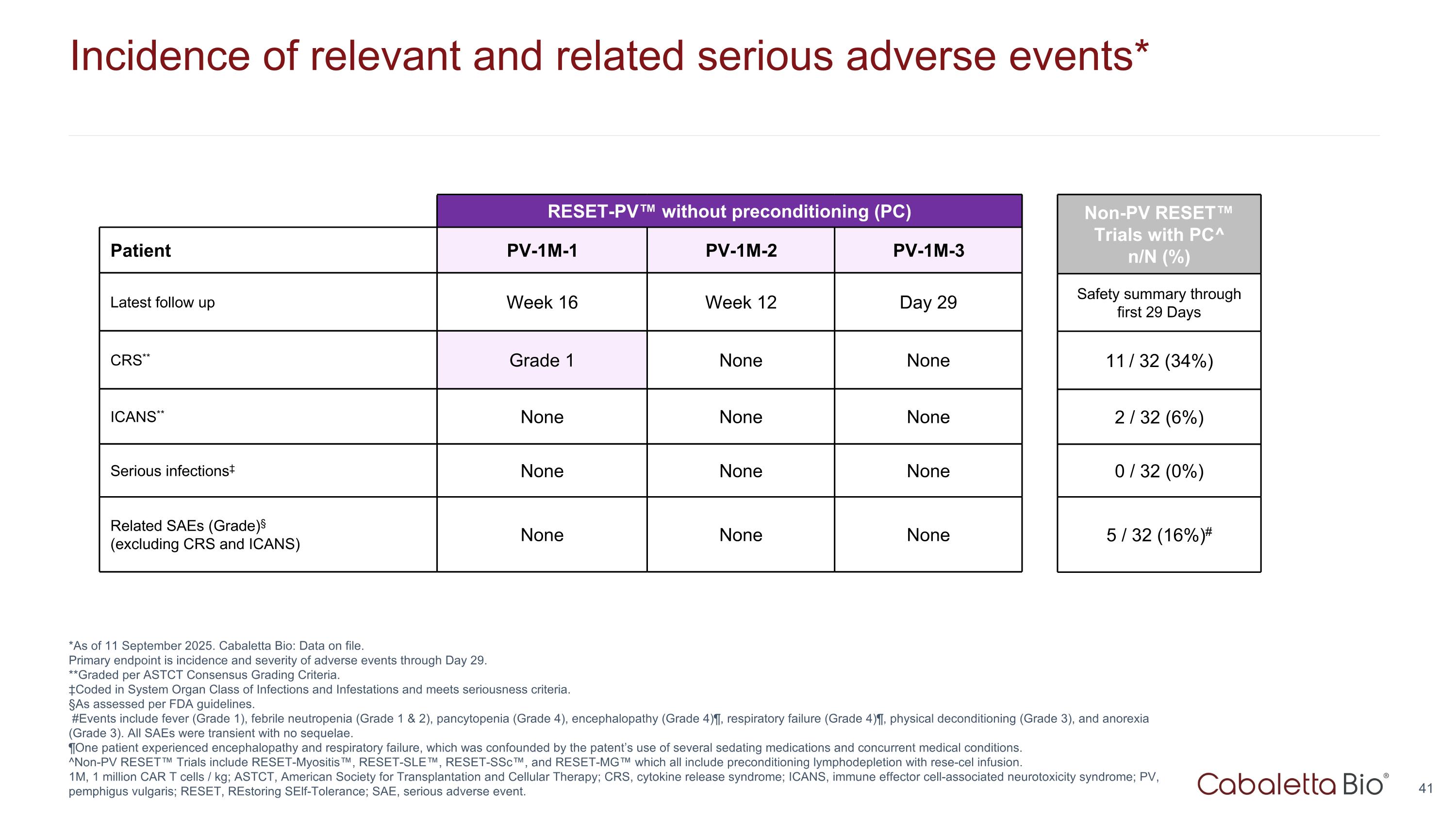
Incidence of relevant and related serious adverse events* *As of 11 September 2025. Cabaletta Bio: Data on file. Primary endpoint is incidence and severity of adverse events through Day 29. **Graded per ASTCT Consensus Grading Criteria. ‡Coded in System Organ Class of Infections and Infestations and meets seriousness criteria. §As assessed per FDA guidelines. #Events include fever (Grade 1), febrile neutropenia (Grade 1 & 2), pancytopenia (Grade 4), encephalopathy (Grade 4)¶, respiratory failure (Grade 4)¶, physical deconditioning (Grade 3), and anorexia (Grade 3). All SAEs were transient with no sequelae. ¶One patient experienced encephalopathy and respiratory failure, which was confounded by the patent’s use of several sedating medications and concurrent medical conditions. ^Non-PV RESET™ Trials include RESET-Myositis™, RESET-SLE™, RESET-SSc™, and RESET-MG™ which all include preconditioning lymphodepletion with rese-cel infusion. 1M, 1 million CAR T cells / kg; ASTCT, American Society for Transplantation and Cellular Therapy; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; PV, pemphigus vulgaris; RESET, REstoring SElf-Tolerance; SAE, serious adverse event. RESET-PV™ without preconditioning (PC) RESET-Myositis Patient PV-1M-1 PV-1M-2 PV-1M-3 Latest follow up Week 16 Week 12 Day 29 CRS** Grade 1 None None ICANS** None None None Serious infections‡ None None None Related SAEs (Grade)§ (excluding CRS and ICANS) None None None Non-PV RESET™ Trials with PC^ n/N (%) Safety summary through first 29 Days 11 / 32 (34%) 2 / 32 (6%) 0 / 32 (0%) 5 / 32 (16%)#
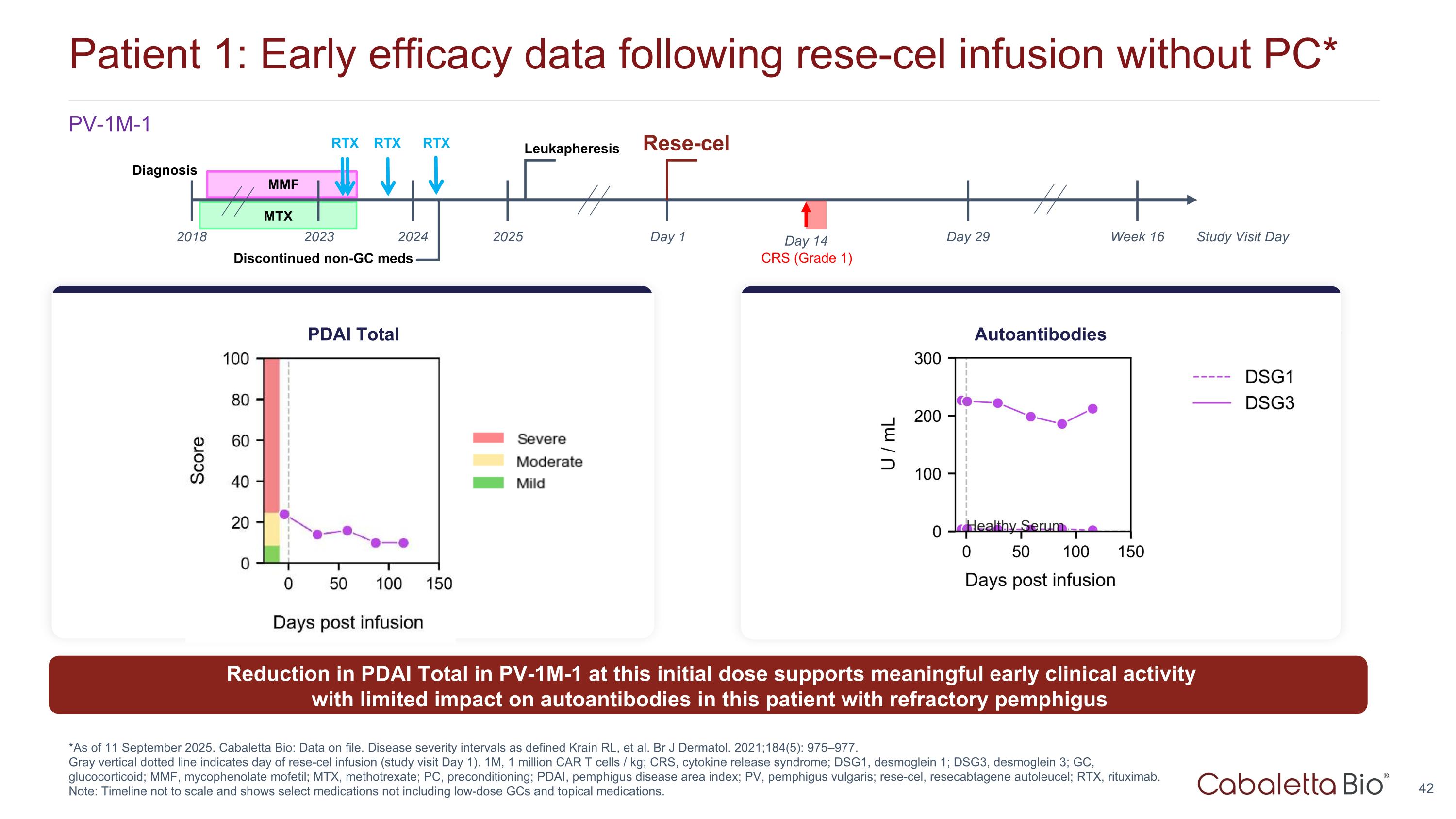
Patient 1: Early efficacy data following rese-cel infusion without PC* *As of 11 September 2025. Cabaletta Bio: Data on file. Disease severity intervals as defined Krain RL, et al. Br J Dermatol. 2021;184(5): 975–977. Gray vertical dotted line indicates day of rese-cel infusion (study visit Day 1). 1M, 1 million CAR T cells / kg; CRS, cytokine release syndrome; DSG1, desmoglein 1; DSG3, desmoglein 3; GC, glucocorticoid; MMF, mycophenolate mofetil; MTX, methotrexate; PC, preconditioning; PDAI, pemphigus disease area index; PV, pemphigus vulgaris; rese-cel, resecabtagene autoleucel; RTX, rituximab. Note: Timeline not to scale and shows select medications not including low-dose GCs and topical medications. Reduction in PDAI Total in PV-1M-1 at this initial dose supports meaningful early clinical activity with limited impact on autoantibodies in this patient with refractory pemphigus PDAI Total Autoantibodies MMF Day 1 Day 29 Rese-cel 2025 2023 Leukapheresis CRS (Grade 1) Study Visit Day Week 16 Discontinued non-GC meds Day 14 2018 Diagnosis 2024 MTX RTX RTX RTX PV-1M-1
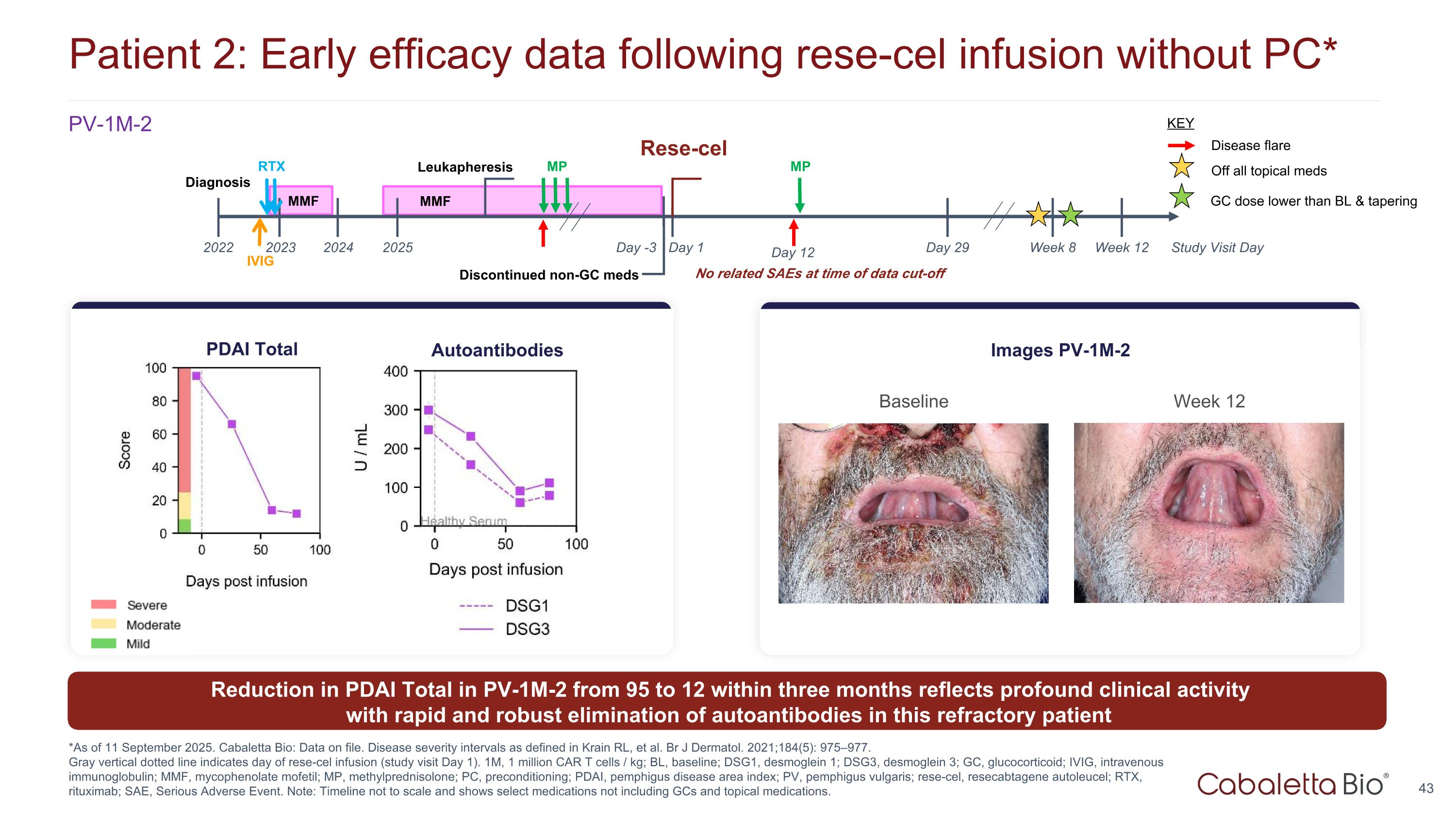
Patient 2: Early efficacy data following rese-cel infusion without PC* *As of 11 September 2025. Cabaletta Bio: Data on file. Disease severity intervals as defined in Krain RL, et al. Br J Dermatol. 2021;184(5): 975–977. Gray vertical dotted line indicates day of rese-cel infusion (study visit Day 1). 1M, 1 million CAR T cells / kg; BL, baseline; DSG1, desmoglein 1; DSG3, desmoglein 3; GC, glucocorticoid; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; MP, methylprednisolone; PC, preconditioning; PDAI, pemphigus disease area index; PV, pemphigus vulgaris; rese-cel, resecabtagene autoleucel; RTX, rituximab; SAE, Serious Adverse Event. Note: Timeline not to scale and shows select medications not including GCs and topical medications. PV-1M-2 Baseline Week 12 Images PV-1M-2 No related SAEs at time of data cut-off Autoantibodies PDAI Total Reduction in PDAI Total in PV-1M-2 from 95 to 12 within three months reflects profound clinical activity with rapid and robust elimination of autoantibodies in this refractory patient MP Day 1 Day 29 2022 2025 2023 Leukapheresis Study Visit Day Week 12 Discontinued non-GC meds MMF Diagnosis Day -3 Day 12 2024 RTX IVIG MMF MP Disease flare KEY Week 8 Off all topical meds GC dose lower than BL & tapering Rese-cel
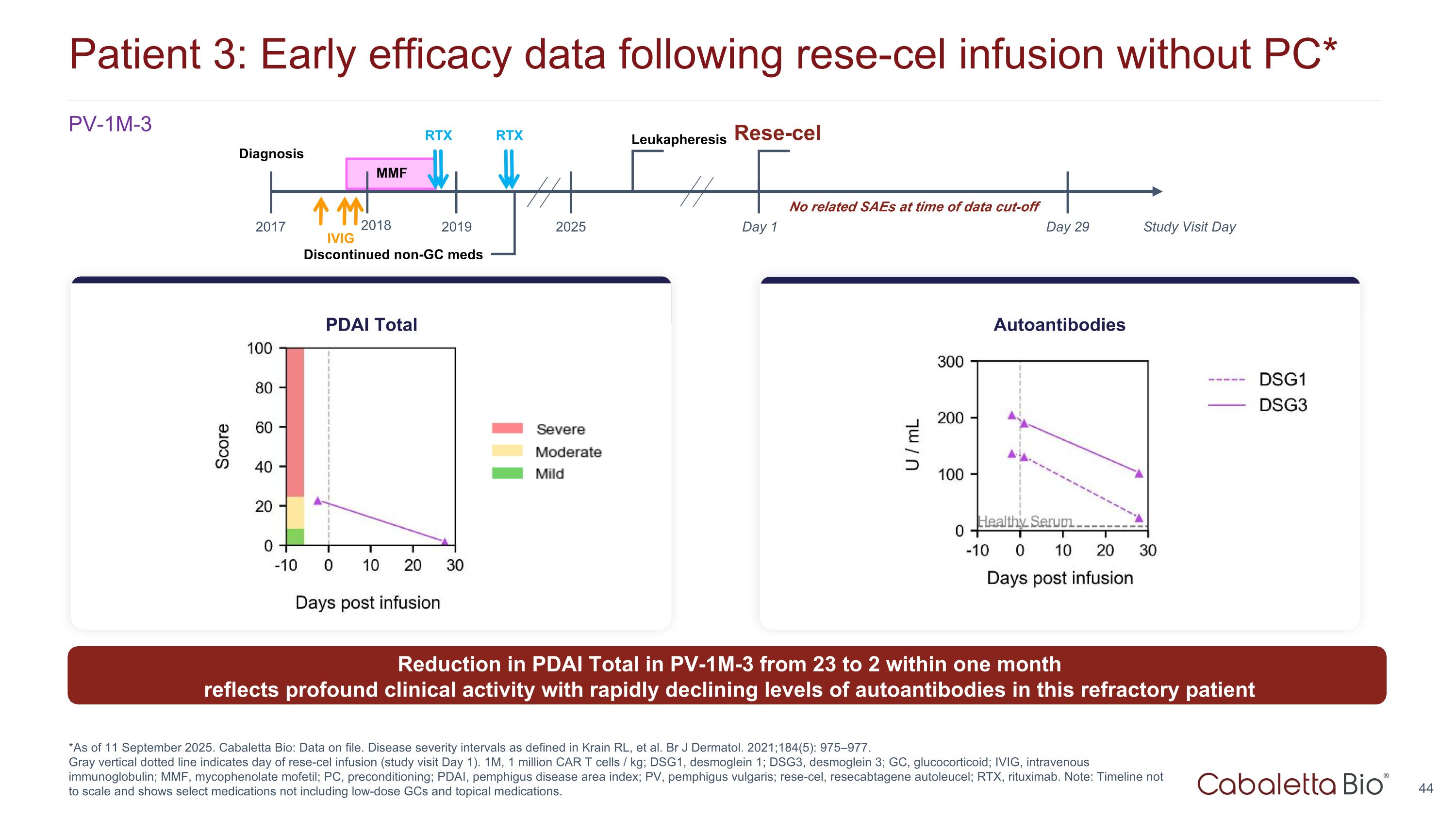
Patient 3: Early efficacy data following rese-cel infusion without PC* *As of 11 September 2025. Cabaletta Bio: Data on file. Disease severity intervals as defined in Krain RL, et al. Br J Dermatol. 2021;184(5): 975–977. Gray vertical dotted line indicates day of rese-cel infusion (study visit Day 1). 1M, 1 million CAR T cells / kg; DSG1, desmoglein 1; DSG3, desmoglein 3; GC, glucocorticoid; IVIG, intravenous immunoglobulin; MMF, mycophenolate mofetil; PC, preconditioning; PDAI, pemphigus disease area index; PV, pemphigus vulgaris; rese-cel, resecabtagene autoleucel; RTX, rituximab. Note: Timeline not to scale and shows select medications not including low-dose GCs and topical medications. PV-1M-3 PDAI Total Autoantibodies Discontinued non-GC meds MMF Day 1 Day 29 2017 2025 2018 Leukapheresis Study Visit Day Diagnosis RTX 2019 RTX IVIG No related SAEs at time of data cut-off Reduction in PDAI Total in PV-1M-3 from 23 to 2 within one month reflects profound clinical activity with rapidly declining levels of autoantibodies in this refractory patient Rese-cel

Rese-cel Manufacturing
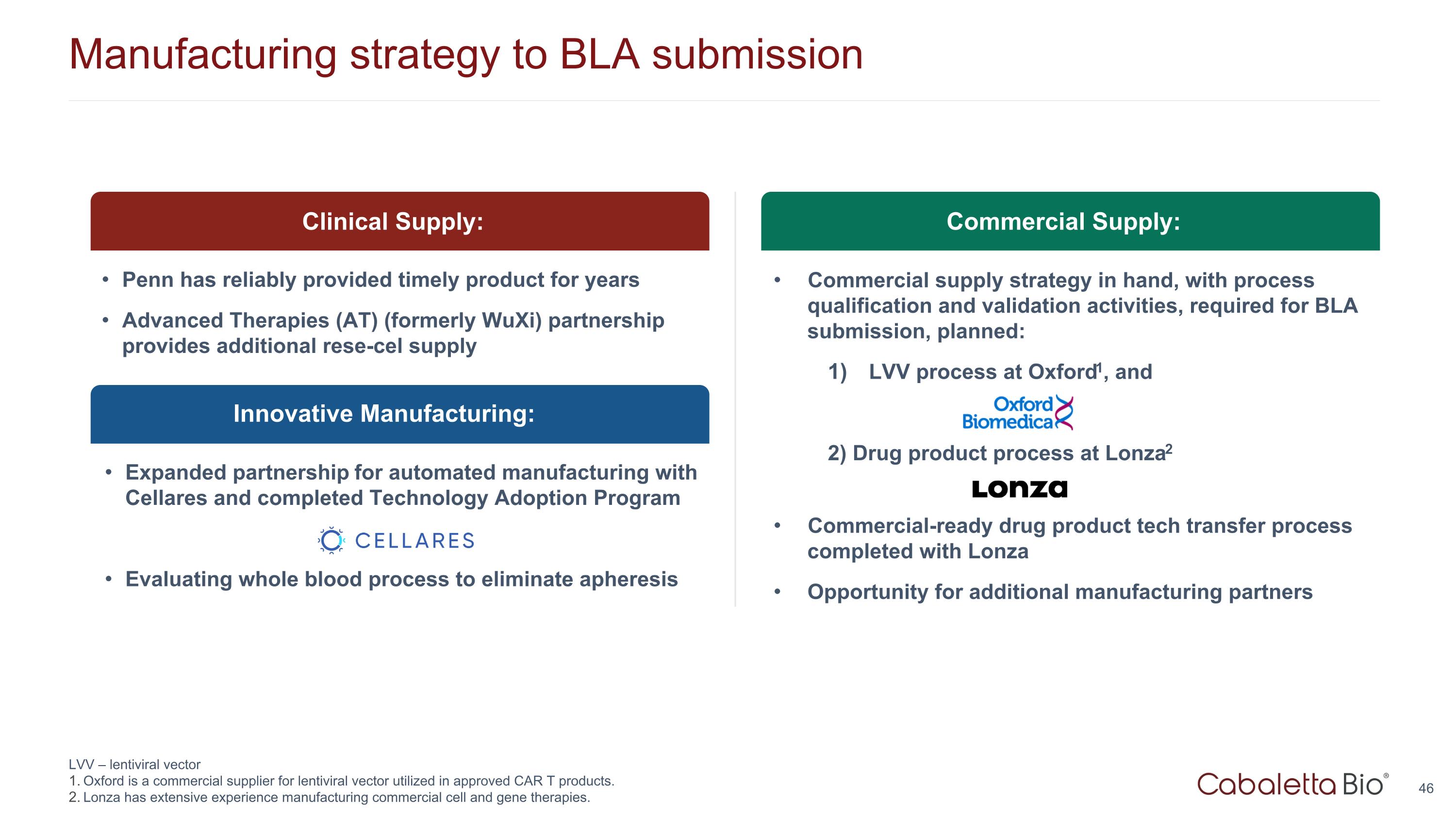
Manufacturing strategy to BLA submission LVV – lentiviral vector Oxford is a commercial supplier for lentiviral vector utilized in approved CAR T products. Lonza has extensive experience manufacturing commercial cell and gene therapies. Innovative Manufacturing: Clinical Supply: Penn has reliably provided timely product for years Advanced Therapies (AT) (formerly WuXi) partnership provides additional rese-cel supply Expanded partnership for automated manufacturing with Cellares and completed Technology Adoption Program Evaluating whole blood process to eliminate apheresis Commercial Supply: Commercial supply strategy in hand, with process qualification and validation activities, required for BLA submission, planned: LVV process at Oxford1, and 2) Drug product process at Lonza2 Commercial-ready drug product tech transfer process completed with Lonza Opportunity for additional manufacturing partners

Corporate Summary

Track record of operational success evaluating & developing novel cell therapy candidates in autoimmunity Cabaletta Bio leadership LEADERSHIP TEAM Anup Marda Chief Financial Officer Nicolette Sherman Chief HR Officer Michael Gerard General Counsel Steven Nichtberger, M.D. President, CEO & Chairman Heather Harte-Hall Chief Compliance Officer Gwendolyn Binder, Ph.D. President, Science & Technology SCIENTIFIC ADVISORY BOARD Aimee Payne, M.D., Ph.D. Co-Founder and Co-Chair Michael C. Milone, M.D., Ph.D. Co-Founder and Co-Chair Carl June, M.D. Jay Siegel, M.D. Drew Weissman, M.D., Ph.D. Iain McInnes, Ph.D., FRCP, FRSE, FMedSci Georg Schett, M.D. David J. Chang, M.D., M.P.H., FACR Chief Medical Officer Samik Basu, M.D. Chief Scientific Officer Arun Das, M.D. Chief Business Officer From Fortune. ©2024 Fortune Media IP Limited. All rights reserved. Used under license. Sarah Yuan Chief Technology Officer

Appendix
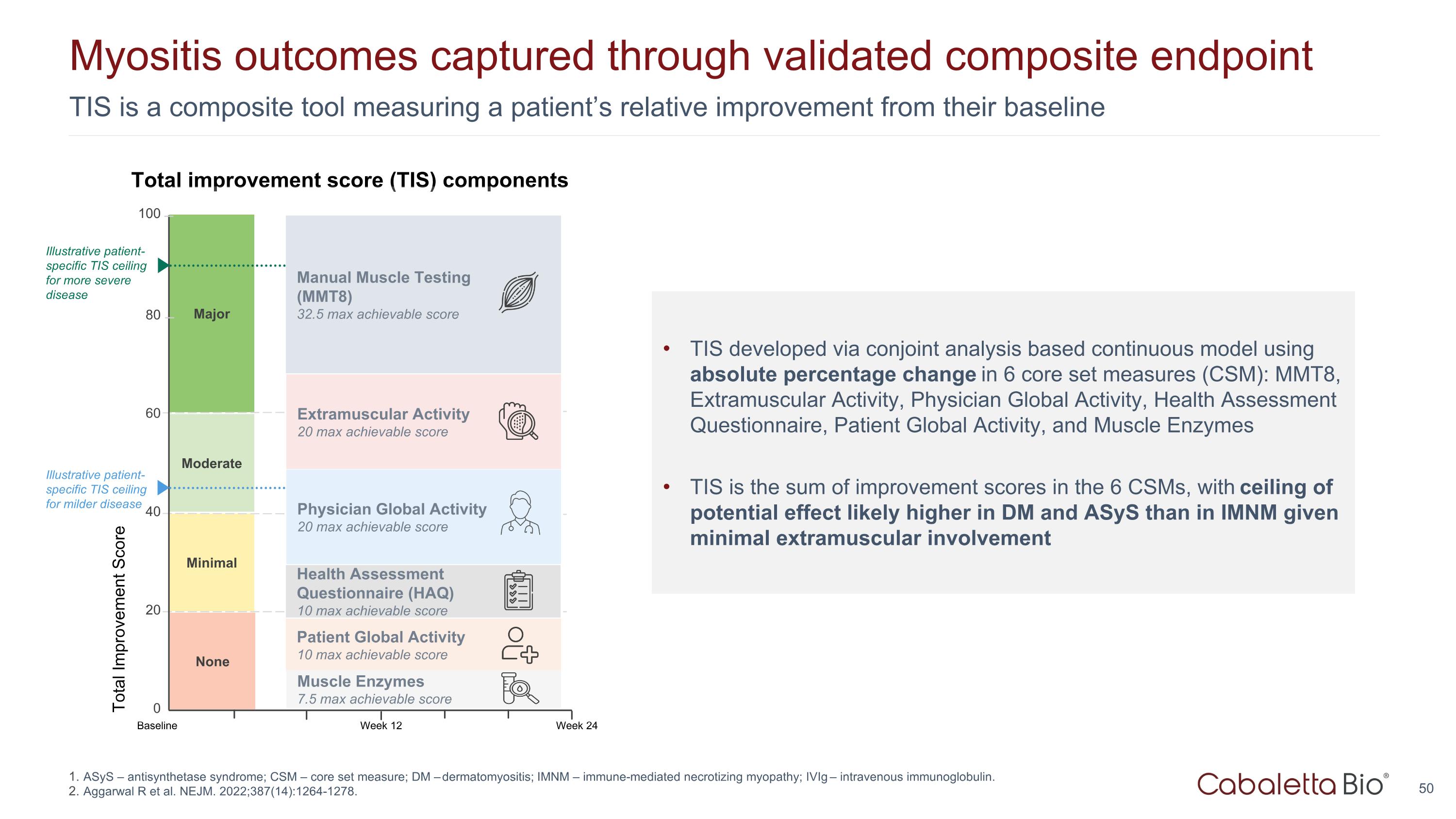
TIS is a composite tool measuring a patient’s relative improvement from their baseline Myositis outcomes captured through validated composite endpoint Major Total Improvement Score 100 80 0 Minimal Moderate 60 40 20 Week 24 Baseline Week 12 None Extramuscular Activity 20 max achievable score Manual Muscle Testing (MMT8) 32.5 max achievable score Patient Global Activity 10 max achievable score Physician Global Activity 20 max achievable score Health Assessment Questionnaire (HAQ) 10 max achievable score Muscle Enzymes 7.5 max achievable score Total improvement score (TIS) components TIS developed via conjoint analysis based continuous model using absolute percentage change in 6 core set measures (CSM): MMT8, Extramuscular Activity, Physician Global Activity, Health Assessment Questionnaire, Patient Global Activity, and Muscle Enzymes TIS is the sum of improvement scores in the 6 CSMs, with ceiling of potential effect likely higher in DM and ASyS than in IMNM given minimal extramuscular involvement Illustrative patient-specific TIS ceiling for milder disease Illustrative patient-specific TIS ceiling for more severe disease ASyS – antisynthetase syndrome; CSM – core set measure; DM – dermatomyositis; IMNM – immune-mediated necrotizing myopathy; IVIg – intravenous immunoglobulin. Aggarwal R et al. NEJM. 2022;387(14):1264-1278.

Corporate Presentation October 2025