

Corporate Presentation march 2023 Exhibit 99.1

Disclaimer The following presentation, including any printed or electronic copy of these slides, the talks given by the presenters, the information communicated during any delivery of the presentation and any question and answer session and any document or material distributed at or in connection with the presentation (collectively, the “Presentation”) has been prepared by Cabaletta Bio, Inc. (“we,” “us,” “our,” “Cabaletta” or the “Company”) and is made for informational purposes only. This Presentation does not purport to be a prospectus, to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this Presentation unless stated otherwise, and this Presentation shall not under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This Presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to our business, operations, and financial conditions, and include, but are not limited to, express or implied statements regarding our current beliefs, expectations and assumptions regarding: our business, future plans and strategies for our CAAR T and CARTA technologies and CABA™ platform; our ability to grow its autoimmune-focused pipeline; the ability to capitalize on and potential benefits resulting from the translational research partnership with Professor Georg Schett and the exclusive license agreement with IASO Bio; the timing of our expected clearance of an Investigational New Drug application (IND) for CABA-201 to the U.S. Food and Drug Administration as well as other planned regulatory filings for our development programs; the progress and results of our DesCAARTes™ Phase 1 trial, including the significance and impact around reported safety and clinical and translational data of cohorts from our DesCAARTes™ trial; the therapeutic potential and clinical benefits of our product candidates; the expectation that Cabaletta may improve outcomes for patients suffering from mucosal pemphigus vulgaris, myasthenia gravis, or other autoimmune diseases; our ability to escalate dosing as high as 10 to 15 billion cells in cohort A6m, initiate dosing in a combination cohort or otherwise; our ability to implement a pre-treatment regimen and the potential ability to enhance in vivo DSG3-CAART exposure; our ability to advance dose escalation in the DesCAARTes™ Phase 1 trial at the current dose ranges for the current cohorts and any projected potential dose ranges for future cohorts, and to optimize our targeted cell therapy; our ability to evaluate, and the potential significance of, the relationship between DSG3-CAART persistence and potential clinical responses in patients with mPV; our ability to safely retreat additional patients and whether we will continue to observe a lack of immune-mediated clearance of DSG3-CAART cells after retreatment and repeat dosing of patients; our ability to successfully complete our preclinical and clinical studies for our product candidates, including CABA-201, our ongoing Phase 1 DesCAARTes™ trial, and our ongoing Phase 1 MusCAARTes™ trial of MuSK-CAART, including our ability to enroll the requisite number of patients, dose each dosing cohort in the intended manner, and progress the trial; the ability of MuSK-CAART to target B cells that differentiate into antibody secreting cells, which produce autoantibodies against muscle-specific kinase; our ability to obtain and maintain regulatory approval of our product candidates, including our expectations regarding the intended incentives conferred by and ability to retain Orphan Drug Designation and Fast Track Designation for DSG3-CAART for the treatment of pemphigus vulgaris and Orphan Drug Designation and Fast Track Designation for MuSK-CAART to improve activities of daily living and muscle strength in patients with MuSK antibody-positive myasthenia gravis; the further expansion and development of our modular CABA™ platform across a range of autoimmune diseases; our ability to contract with third-party suppliers and manufacturers, implement an enhanced manufacturing process and further develop our internal manufacturing strategy, capabilities and facilities; our potential commercial opportunities, including value and addressable market, for our product candidates; our expectations regarding our use of capital and other financial results; and our ability to fund operations into the first quarter of 2025. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Various risks, uncertainties and assumptions could cause actual results to differ materially from those anticipated or implied in our forward-looking statements. Such risks and uncertainties include, but are not limited to, risks related to the success, cost, and timing of our product candidate development activities and preclinical studies and clinical trials, risks related to our ability to demonstrate sufficient evidence of safety, efficacy and tolerability in our preclinical studies and clinical trials of CABA-201, DSG3-CAART and MuSK-CAART, the risk that the results observed with the similarly-designed construct employed in the recent Nature Medicine publication are not indicative of the results we seek to achieve with CABA-201, our plans to evaluate additional cohorts in the DesCAARTes™ trial, including a cohort implementing a pre-treatment regimen, the risk that signs of biologic activity or persistence may not inform long-term results, the risk that persistence observed with effective CART-19 oncology studies in combination with lymphodepletion is not indicative of, or applicable to, clinical responses in patients with mPV, risks related to clinical trial site activation or enrollment rates that are lower than expected, our ability to protect and maintain our intellectual property position, risks related to our relationship with third parties, uncertainties related to regulatory agencies’ evaluation of regulatory filings and other information related to our product candidates, our ability to retain and recognize the intended incentives conferred by any Orphan Drug Designation and Fast Track Designations, the risk that any one or more of our product candidates will not be successfully developed and commercialized, the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies, the impact of COVID-19 on the timing, progress, interpretability of data, and results of ongoing or planned clinical trials and risks relating to as a result of extraordinary events or circumstances such as the COVID-19 pandemic, and any business interruptions to our operations or to those of our clinical sites, manufacturers, suppliers, or other vendors resulting from the COVID-19 pandemic or similar public health crisis. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ materially from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our most recent annual report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in our other filings with the Securities and Exchange Commission. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. The Company is the owner of various trademarks, trade names and service marks. Certain other trademarks, trade names and service marks appearing in this Presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this Presentation are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.

Develop and launch the first curative targeted cellular therapies for patients with autoimmune diseases
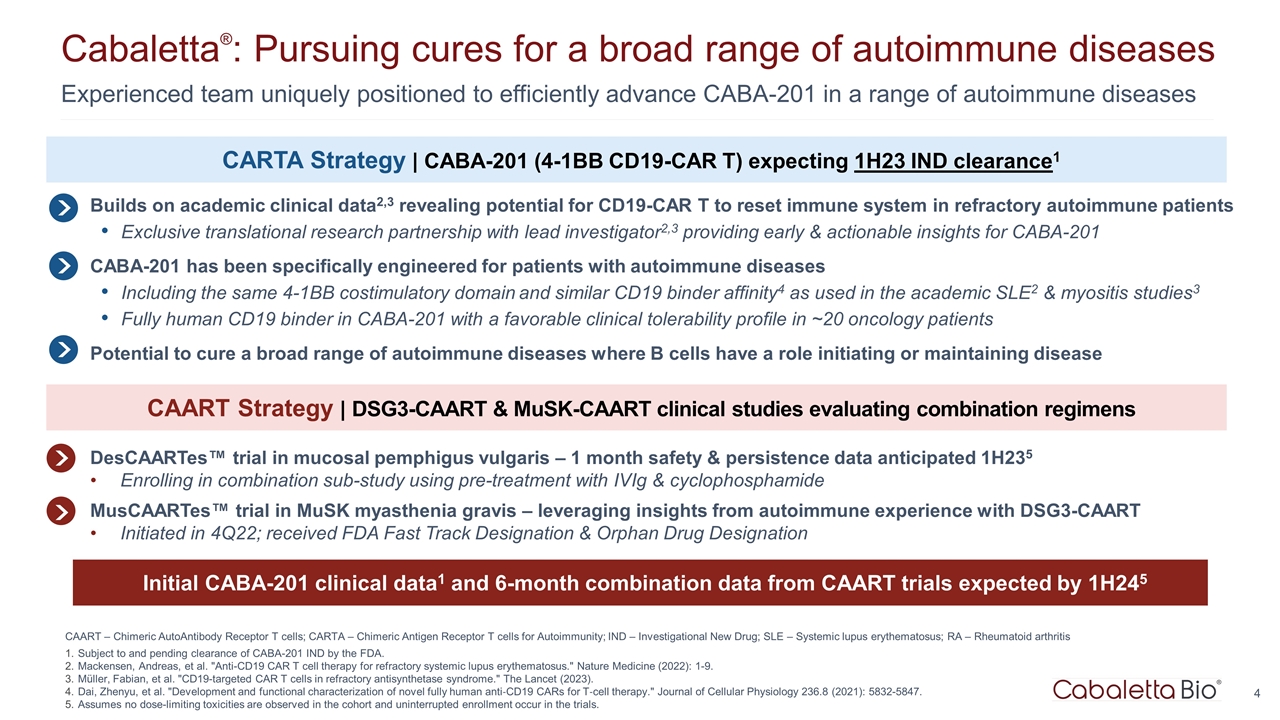
Experienced team uniquely positioned to efficiently advance CABA-201 in a range of autoimmune diseases Cabaletta®: Pursuing cures for a broad range of autoimmune diseases CAART – Chimeric AutoAntibody Receptor T cells; CARTA – Chimeric Antigen Receptor T cells for Autoimmunity; IND – Investigational New Drug; SLE – Systemic lupus erythematosus; RA – Rheumatoid arthritis Subject to and pending clearance of CABA-201 IND by the FDA. Mackensen, Andreas, et al. "Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus." Nature Medicine (2022): 1-9. Müller, Fabian, et al. "CD19-targeted CAR T cells in refractory antisynthetase syndrome." The Lancet (2023). Dai, Zhenyu, et al. "Development and functional characterization of novel fully human anti‐CD19 CARs for T‐cell therapy." Journal of Cellular Physiology 236.8 (2021): 5832-5847. Assumes no dose-limiting toxicities are observed in the cohort and uninterrupted enrollment occur in the trials. Builds on academic clinical data2,3 revealing potential for CD19-CAR T to reset immune system in refractory autoimmune patients Exclusive translational research partnership with lead investigator2,3 providing early & actionable insights for CABA-201 CABA-201 has been specifically engineered for patients with autoimmune diseases Including the same 4-1BB costimulatory domain and similar CD19 binder affinity4 as used in the academic SLE2 & myositis studies3 Fully human CD19 binder in CABA-201 with a favorable clinical tolerability profile in ~20 oncology patients Potential to cure a broad range of autoimmune diseases where B cells have a role initiating or maintaining disease DesCAARTes™ trial in mucosal pemphigus vulgaris – 1 month safety & persistence data anticipated 1H235 Enrolling in combination sub-study using pre-treatment with IVIg & cyclophosphamide MusCAARTes™ trial in MuSK myasthenia gravis – leveraging insights from autoimmune experience with DSG3-CAART Initiated in 4Q22; received FDA Fast Track Designation & Orphan Drug Designation CARTA Strategy | CABA-201 (4-1BB CD19-CAR T) expecting 1H23 IND clearance1 CAART Strategy | DSG3-CAART & MuSK-CAART clinical studies evaluating combination regimens Initial CABA-201 clinical data1 and 6-month combination data from CAART trials expected by 1H245
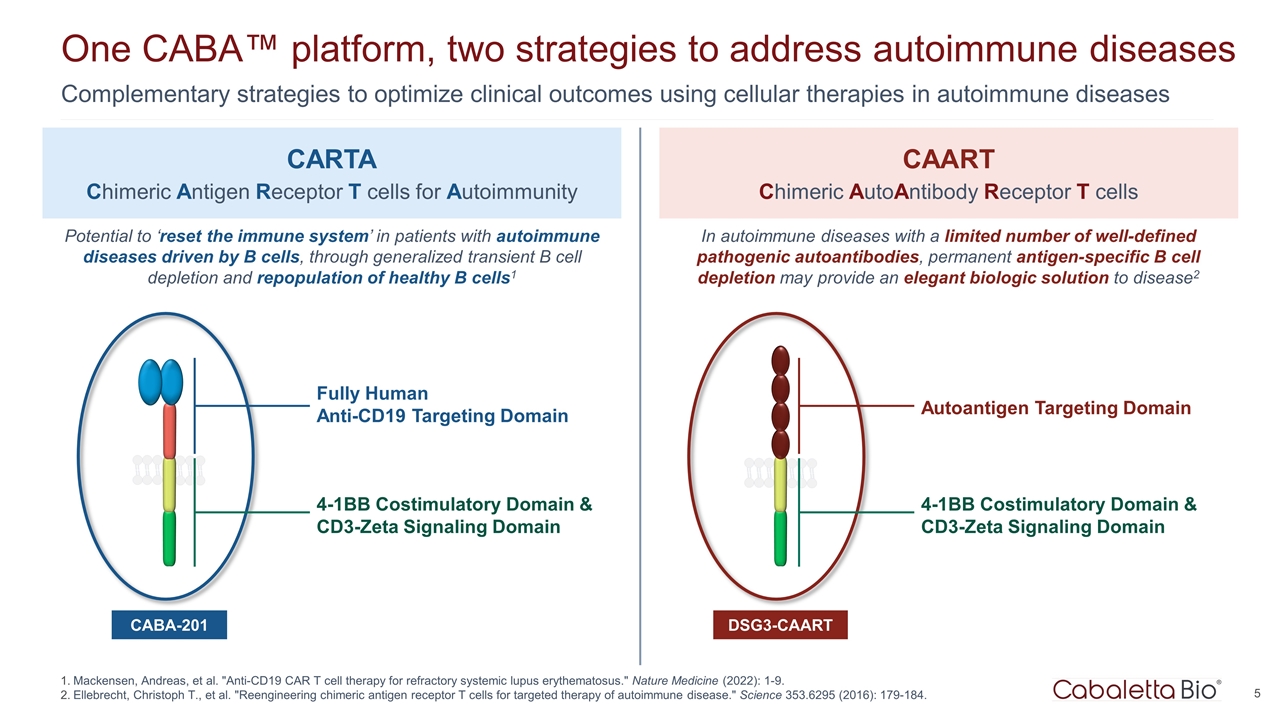
One CABA™ platform, two strategies to address autoimmune diseases Complementary strategies to optimize clinical outcomes using cellular therapies in autoimmune diseases Mackensen, Andreas, et al. "Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus." Nature Medicine (2022): 1-9. Ellebrecht, Christoph T., et al. "Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease." Science 353.6295 (2016): 179-184. Fully Human Anti-CD19 Targeting Domain CARTA Chimeric Antigen Receptor T cells for Autoimmunity CAART Chimeric AutoAntibody Receptor T cells CABA-201 4-1BB Costimulatory Domain & CD3-Zeta Signaling Domain DSG3-CAART Potential to ‘reset the immune system’ in patients with autoimmune diseases driven by B cells, through generalized transient B cell depletion and repopulation of healthy B cells1 In autoimmune diseases with a limited number of well-defined pathogenic autoantibodies, permanent antigen-specific B cell depletion may provide an elegant biologic solution to disease2 Autoantigen Targeting Domain 4-1BB Costimulatory Domain & CD3-Zeta Signaling Domain
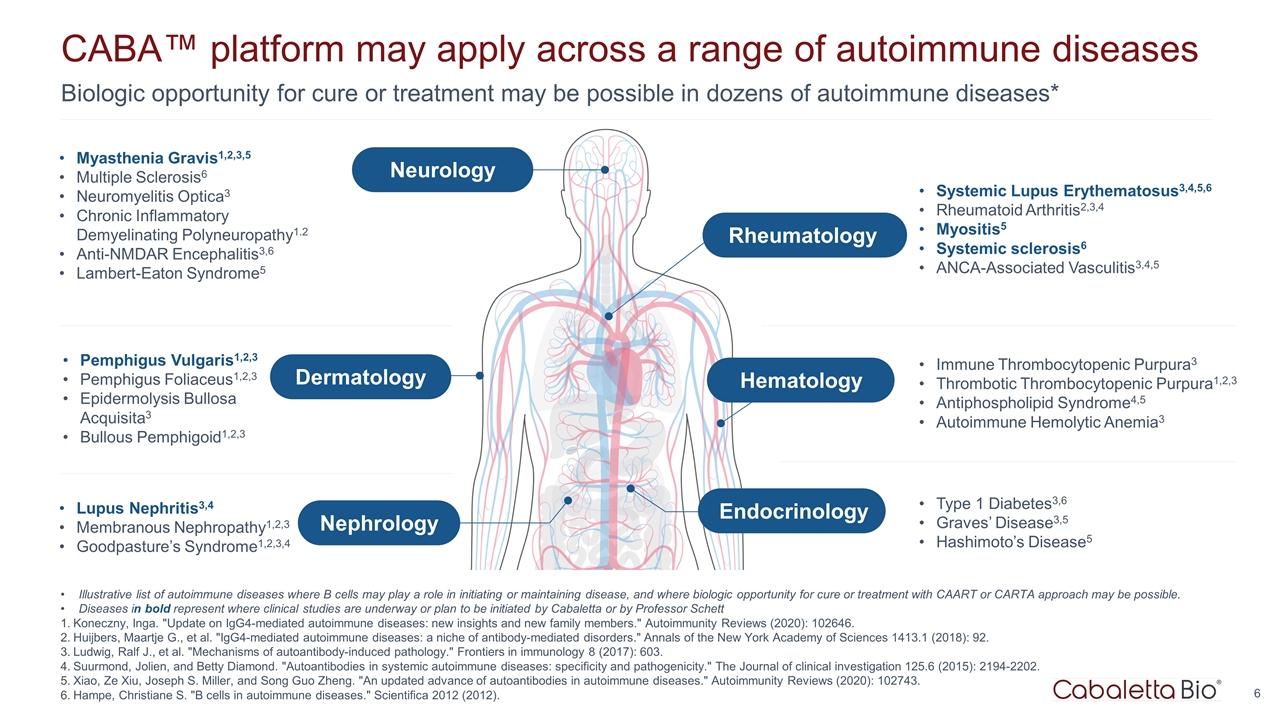
Biologic opportunity for cure or treatment may be possible in dozens of autoimmune diseases* CABA™ platform may apply across a range of autoimmune diseases Pemphigus Vulgaris1,2,3 Pemphigus Foliaceus1,2,3 Epidermolysis Bullosa Acquisita3 Bullous Pemphigoid1,2,3 Lupus Nephritis3,4 Membranous Nephropathy1,2,3 Goodpasture’s Syndrome1,2,3,4 Myasthenia Gravis1,2,3,5 Multiple Sclerosis6 Neuromyelitis Optica3 Chronic Inflammatory Demyelinating Polyneuropathy1.2 Anti-NMDAR Encephalitis3,6 Lambert-Eaton Syndrome5 Systemic Lupus Erythematosus3,4,5,6 Rheumatoid Arthritis2,3,4 Myositis5 Systemic sclerosis6 ANCA-Associated Vasculitis3,4,5 Immune Thrombocytopenic Purpura3 Thrombotic Thrombocytopenic Purpura1,2,3 Antiphospholipid Syndrome4,5 Autoimmune Hemolytic Anemia3 Type 1 Diabetes3,6 Graves’ Disease3,5 Hashimoto’s Disease5 Illustrative list of autoimmune diseases where B cells may play a role in initiating or maintaining disease, and where biologic opportunity for cure or treatment with CAART or CARTA approach may be possible. Diseases in bold represent where clinical studies are underway or plan to be initiated by Cabaletta or by Professor Schett Koneczny, Inga. "Update on IgG4-mediated autoimmune diseases: new insights and new family members." Autoimmunity Reviews (2020): 102646. Huijbers, Maartje G., et al. "IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders." Annals of the New York Academy of Sciences 1413.1 (2018): 92. Ludwig, Ralf J., et al. "Mechanisms of autoantibody-induced pathology." Frontiers in immunology 8 (2017): 603. Suurmond, Jolien, and Betty Diamond. "Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity." The Journal of clinical investigation 125.6 (2015): 2194-2202. Xiao, Ze Xiu, Joseph S. Miller, and Song Guo Zheng. "An updated advance of autoantibodies in autoimmune diseases." Autoimmunity Reviews (2020): 102743. Hampe, Christiane S. "B cells in autoimmune diseases." Scientifica 2012 (2012). Dermatology Nephrology Neurology Rheumatology Hematology Endocrinology
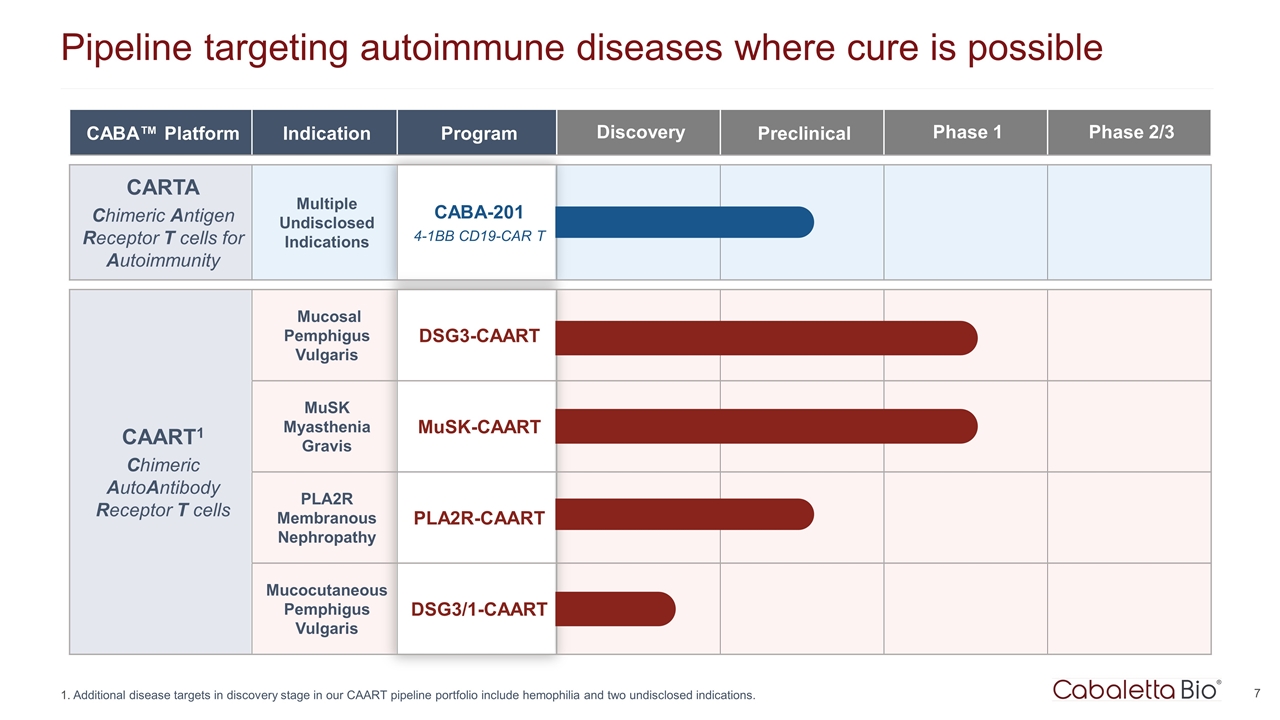
Pipeline targeting autoimmune diseases where cure is possible Additional disease targets in discovery stage in our CAART pipeline portfolio include hemophilia and two undisclosed indications. CABA™ Platform Indication Program Discovery Preclinical Phase 1 Phase 2/3 CARTA Chimeric Antigen Receptor T cells for Autoimmunity Multiple Undisclosed Indications CABA-201 4-1BB CD19-CAR T CAART1 Chimeric AutoAntibody Receptor T cells Mucosal Pemphigus Vulgaris DSG3-CAART MuSK Myasthenia Gravis MuSK-CAART PLA2R Membranous Nephropathy PLA2R-CAART Mucocutaneous Pemphigus Vulgaris DSG3/1-CAART

Chimeric Antigen Receptor T Cells for Autoimmunity CABA-201
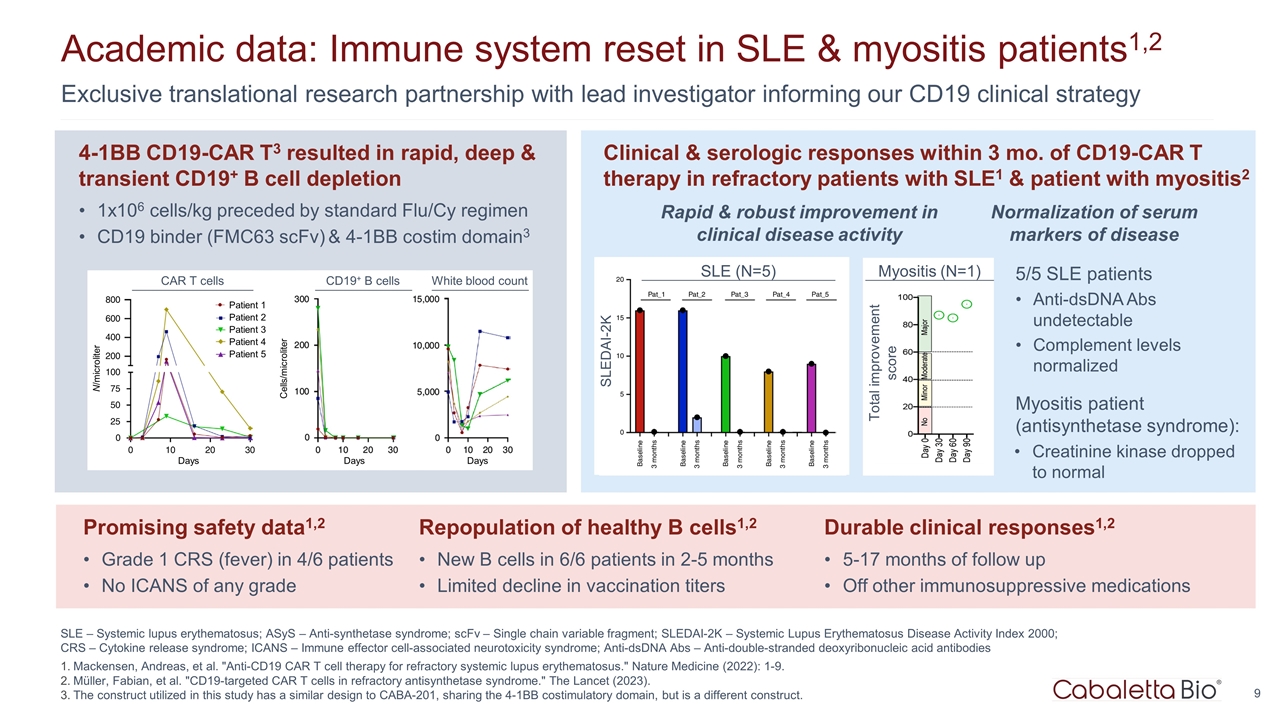
SLE – Systemic lupus erythematosus; ASyS – Anti-synthetase syndrome; scFv – Single chain variable fragment; SLEDAI-2K – Systemic Lupus Erythematosus Disease Activity Index 2000; CRS – Cytokine release syndrome; ICANS – Immune effector cell-associated neurotoxicity syndrome; Anti-dsDNA Abs – Anti-double-stranded deoxyribonucleic acid antibodies Mackensen, Andreas, et al. "Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus." Nature Medicine (2022): 1-9. Müller, Fabian, et al. "CD19-targeted CAR T cells in refractory antisynthetase syndrome." The Lancet (2023). The construct utilized in this study has a similar design to CABA-201, sharing the 4-1BB costimulatory domain, but is a different construct. Exclusive translational research partnership with lead investigator informing our CD19 clinical strategy Academic data: Immune system reset in SLE & myositis patients1,2 5/5 SLE patients Anti-dsDNA Abs undetectable Complement levels normalized Myositis patient (antisynthetase syndrome): Creatinine kinase dropped to normal Durable clinical responses1,2 5-17 months of follow up Off other immunosuppressive medications CAR T cells CD19+ B cells White blood count Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 Days Days Days Cells/microliter N/microliter Promising safety data1,2 Grade 1 CRS (fever) in 4/6 patients No ICANS of any grade 4-1BB CD19-CAR T3 resulted in rapid, deep & transient CD19+ B cell depletion 1x106 cells/kg preceded by standard Flu/Cy regimen CD19 binder (FMC63 scFv) & 4-1BB costim domain3 Clinical & serologic responses within 3 mo. of CD19-CAR T therapy in refractory patients with SLE1 & patient with myositis2 Repopulation of healthy B cells1,2 New B cells in 6/6 patients in 2-5 months Limited decline in vaccination titers Rapid & robust improvement in clinical disease activity Normalization of serum markers of disease SLE (N=5) SLEDAI-2K Myositis (N=1) Total improvement score
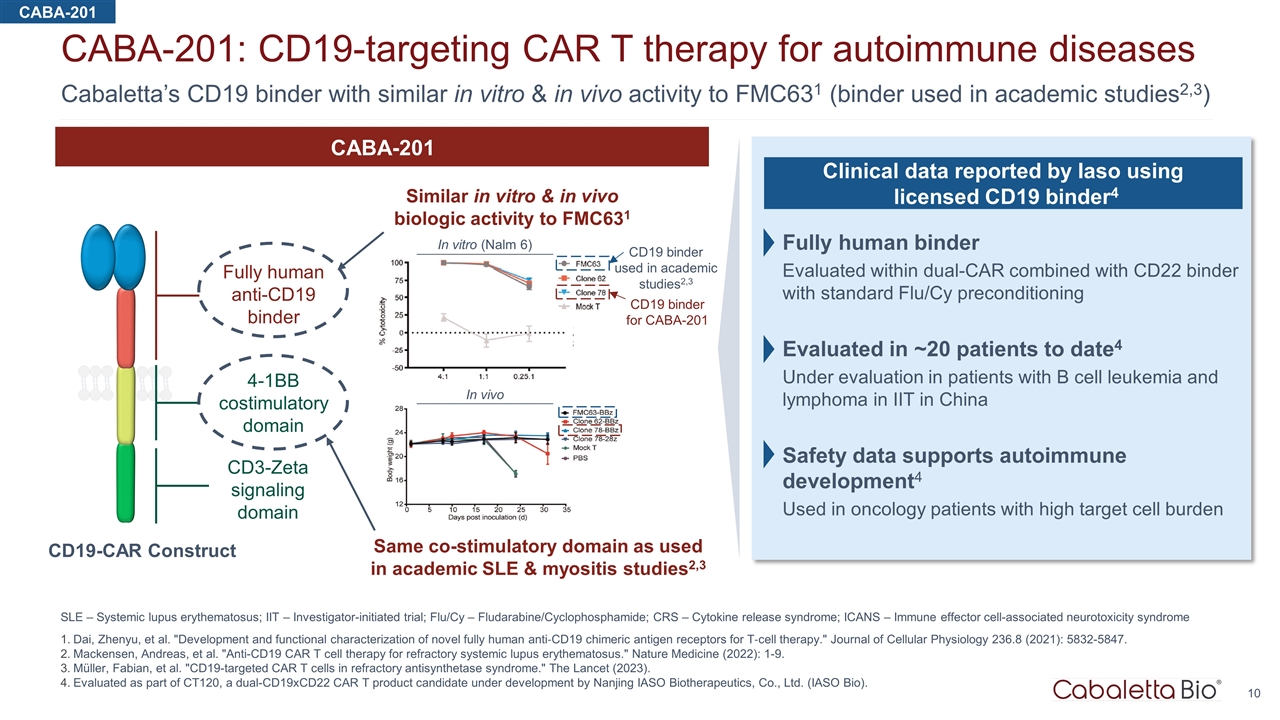
Cabaletta’s CD19 binder with similar in vitro & in vivo activity to FMC631 (binder used in academic studies2,3) CABA-201: CD19-targeting CAR T therapy for autoimmune diseases SLE – Systemic lupus erythematosus; IIT – Investigator-initiated trial; Flu/Cy – Fludarabine/Cyclophosphamide; CRS – Cytokine release syndrome; ICANS – Immune effector cell-associated neurotoxicity syndrome Dai, Zhenyu, et al. "Development and functional characterization of novel fully human anti‐CD19 chimeric antigen receptors for T‐cell therapy." Journal of Cellular Physiology 236.8 (2021): 5832-5847. Mackensen, Andreas, et al. "Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus." Nature Medicine (2022): 1-9. Müller, Fabian, et al. "CD19-targeted CAR T cells in refractory antisynthetase syndrome." The Lancet (2023). Evaluated as part of CT120, a dual-CD19xCD22 CAR T product candidate under development by Nanjing IASO Biotherapeutics, Co., Ltd. (IASO Bio). CD19 binder for CABA-201 CD19 binder used in academic studies2,3 In vitro (Nalm 6) In vivo Fully human anti-CD19 binder 4-1BB costimulatory domain CD3-Zeta signaling domain CABA-201 Similar in vitro & in vivo biologic activity to FMC631 Same co-stimulatory domain as used in academic SLE & myositis studies2,3 CD19-CAR Construct Clinical data reported by Iaso using licensed CD19 binder4 Fully human binder Evaluated within dual-CAR combined with CD22 binder with standard Flu/Cy preconditioning Evaluated in ~20 patients to date4 Under evaluation in patients with B cell leukemia and lymphoma in IIT in China Safety data supports autoimmune development4 Used in oncology patients with high target cell burden CABA-201
Differentiated asset, exclusive translational insights & track record of timely CAART clinical implementation Accelerating development of CABA-201 for autoimmune diseases SLE – Systemic lupus erythematosus Mackensen, Andreas, et al. "Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus." Nature Medicine (2022): 1-9. Müller, Fabian, et al. "CD19-targeted CAR T cells in refractory antisynthetase syndrome." The Lancet (2023). IND clearance for CABA-201 expected in first half of 2023 1 Cabaletta is uniquely positioned to efficiently advance CABA-201 in autoimmune diseases Expertise in conducting complex, interdisciplinary autoimmune-focused cell therapy clinical trials Track record of positive regulatory interactions to support cell therapy trials in autoimmune diseases since 2018 2 INDs cleared within routine 30-day period; multiple clinical protocol modifications, Fast Track designations granted Successful track record of manufacturing novel cell therapy products with academic and industry partners Deep understanding of autoimmunity allows potential application across broad range of diseases 2 Clinically-evaluated binder & translational research partnership support CABA-201 development CABA-201 leverages similar design to 4-1BB-containing CD19-CAR T in seminal academic SLE & myositis studies1,2 Exclusive research partnership with lead investigator for academic studies provides early and actionable insights CABA-201

Chimeric AutoAntibody Receptor T Cells DSG3-CAART & MuSK-CAART
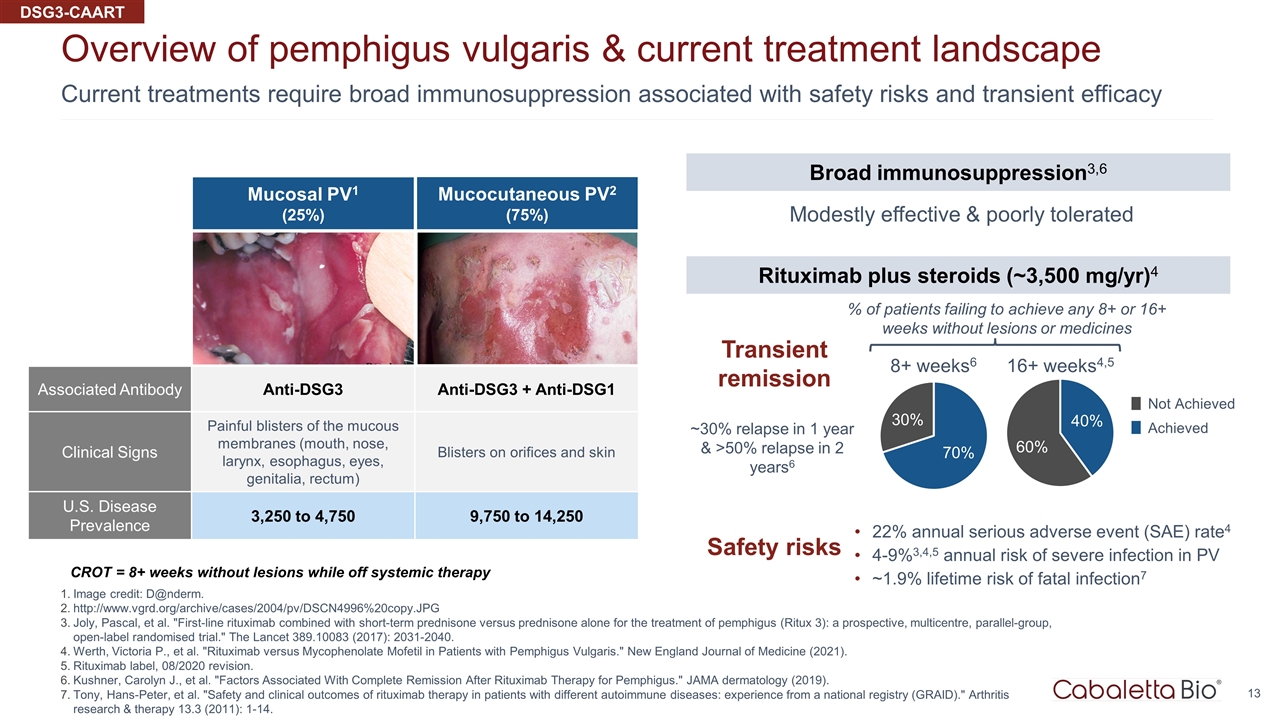
Current treatments require broad immunosuppression associated with safety risks and transient efficacy Overview of pemphigus vulgaris & current treatment landscape Image credit: D@nderm. http://www.vgrd.org/archive/cases/2004/pv/DSCN4996%20copy.JPG Joly, Pascal, et al. "First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial." The Lancet 389.10083 (2017): 2031-2040. Werth, Victoria P., et al. "Rituximab versus Mycophenolate Mofetil in Patients with Pemphigus Vulgaris." New England Journal of Medicine (2021). Rituximab label, 08/2020 revision. Kushner, Carolyn J., et al. "Factors Associated With Complete Remission After Rituximab Therapy for Pemphigus." JAMA dermatology (2019). Tony, Hans-Peter, et al. "Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID)." Arthritis research & therapy 13.3 (2011): 1-14. CROT = 8+ weeks without lesions while off systemic therapy Mucosal PV1 (25%) Associated Antibody Anti-DSG3 Anti-DSG3 + Anti-DSG1 Clinical Signs Painful blisters of the mucous membranes (mouth, nose, larynx, esophagus, eyes, genitalia, rectum) Blisters on orifices and skin U.S. Disease Prevalence 3,250 to 4,750 9,750 to 14,250 Mucocutaneous PV2 (75%) DSG3-CAART Transient remission Broad immunosuppression3,6 Rituximab plus steroids (~3,500 mg/yr)4 Modestly effective & poorly tolerated 8+ weeks6 16+ weeks4,5 % of patients failing to achieve any 8+ or 16+ weeks without lesions or medicines Not Achieved Achieved ~30% relapse in 1 year & >50% relapse in 2 years6 Safety risks 22% annual serious adverse event (SAE) rate4 4-9%3,4,5 annual risk of severe infection in PV ~1.9% lifetime risk of fatal infection7
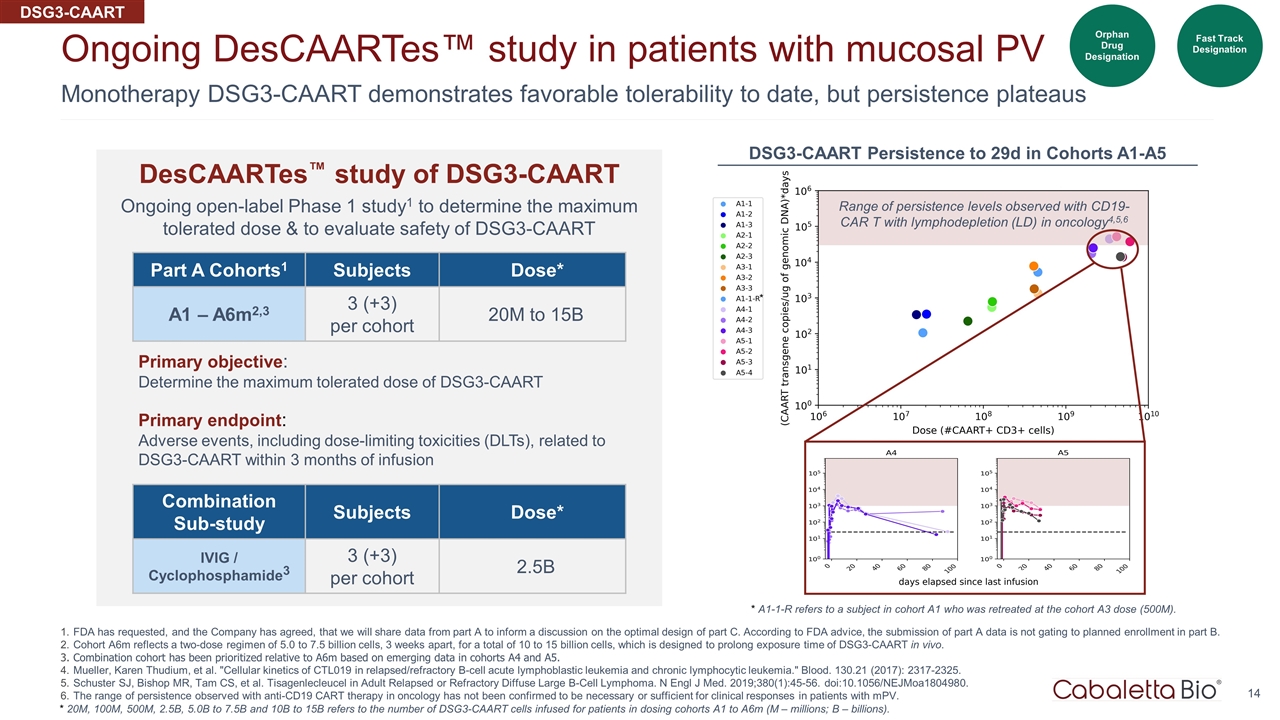
Monotherapy DSG3-CAART demonstrates favorable tolerability to date, but persistence plateaus Ongoing DesCAARTes™ study in patients with mucosal PV FDA has requested, and the Company has agreed, that we will share data from part A to inform a discussion on the optimal design of part C. According to FDA advice, the submission of part A data is not gating to planned enrollment in part B. Cohort A6m reflects a two-dose regimen of 5.0 to 7.5 billion cells, 3 weeks apart, for a total of 10 to 15 billion cells, which is designed to prolong exposure time of DSG3-CAART in vivo. Combination cohort has been prioritized relative to A6m based on emerging data in cohorts A4 and A5. Mueller, Karen Thudium, et al. "Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia." Blood. 130.21 (2017): 2317-2325. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45-56. doi:10.1056/NEJMoa1804980. The range of persistence observed with anti-CD19 CART therapy in oncology has not been confirmed to be necessary or sufficient for clinical responses in patients with mPV. Fast Track Designation Part A Cohorts1 Subjects Dose* A1 – A6m2,3 3 (+3) per cohort 20M to 15B Primary objective: Determine the maximum tolerated dose of DSG3-CAART Primary endpoint: Adverse events, including dose-limiting toxicities (DLTs), related to DSG3-CAART within 3 months of infusion * 20M, 100M, 500M, 2.5B, 5.0B to 7.5B and 10B to 15B refers to the number of DSG3-CAART cells infused for patients in dosing cohorts A1 to A6m (M – millions; B – billions). Combination Sub-study Subjects Dose* IVIG / Cyclophosphamide3 3 (+3) per cohort 2.5B DSG3-CAART DesCAARTes™ study of DSG3-CAART Ongoing open-label Phase 1 study1 to determine the maximum tolerated dose & to evaluate safety of DSG3-CAART Range of persistence levels observed with CD19-CAR T with lymphodepletion (LD) in oncology4,5,6 * DSG3-CAART Persistence to 29d in Cohorts A1-A5 Fast Track Designation Orphan Drug Designation * A1-1-R refers to a subject in cohort A1 who was retreated at the cohort A3 dose (500M).
Combination strategy designed to enhance cytokine and/or diminish autoantibody effects on CAART activity Combination sub-study prioritized to increase CAART exposure Amagai, Masayuki, et al. "A randomized double-blind trial of intravenous immunoglobulin for pemphigus." Journal of the American Academy of Dermatology 60.4 (2009): 595-603. Arnold, D. F., et al. "An ‘n‐of‐1’placebo‐controlled crossover trial of IVIg as adjuvant therapy in refractory pemphigus vulgaris." British Journal of Dermatology 160.5 (2009): 1098-1102. Zhang, Wenjing, et al. "Short-Term Intravenous Infusion of Cyclophosphamide in the Treatment of Refractory Pemphigus Vulgaris: A Retrospective Study." Dermatology 237.2 (2021): 185-190. Fleischli, Mary E., Rachel H. Valek, and Amit G. Pandya. "Pulse intravenous cyclophosphamide therapy in pemphigus." Archives of dermatology 135.1 (1999): 57-61. Lolis, Margarita, et al. "Effect of IVIg with or without cytotoxic drugs on pemphigus intercellular antibodies." Journal of the American Academy of Dermatology 64.3 (2011): 484-489. Combination sub-study cohort A4 dose (2.5x109 cells) + cyclophosphamide (CY) & IVIg Dose-dependent increase in CAART persistence as monotherapy plateaued with Cohort A5 Through up to 6 months post-CAART infusion, no clear pattern in antibody levels and disease activity observed in first 3 subjects at cohort A5 dose CY may reduce ‘cytokine sink,’ potentially enhancing CAART activation & proliferation CY + IVIg may reduce anti-DSG3 autoantibodies, addressing a potential efficacy barrier CY & IVIg likely to provide transient improvement in first few months after infusion1,2,3,4,5 DSG3-CAART clinical effect may require follow-up for 6-9 months Cohort A6m | 2x A5 dose (1-1.5x1010 cells) – lower priority Two infusions at the A5 dose level 3 weeks apart To potentially increase the duration of maximal exposure to DSG3-CAART DSG3-CAART
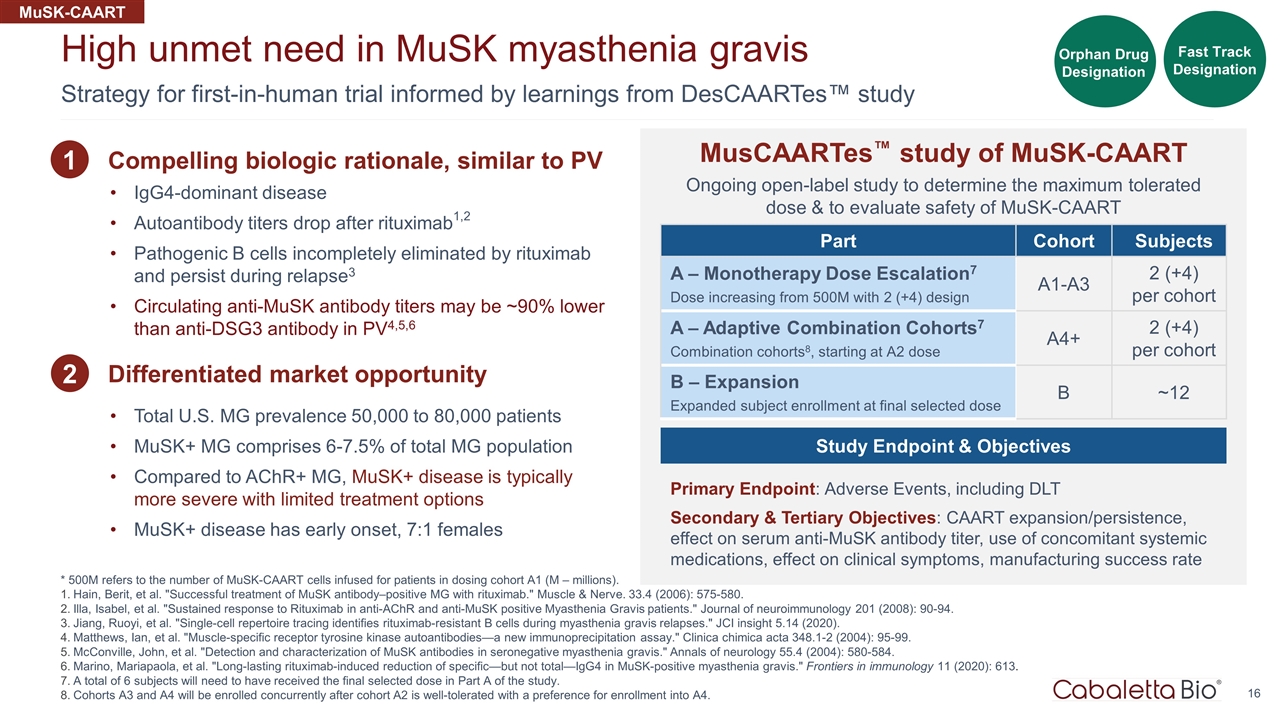
Strategy for first-in-human trial informed by learnings from DesCAARTes™ study High unmet need in MuSK myasthenia gravis MusCAARTes™ study of MuSK-CAART Ongoing open-label study to determine the maximum tolerated dose & to evaluate safety of MuSK-CAART Part Cohort Subjects A – Monotherapy Dose Escalation7 Dose increasing from 500M with 2 (+4) design A1-A3 2 (+4) per cohort A – Adaptive Combination Cohorts7 Combination cohorts8, starting at A2 dose A4+ 2 (+4) per cohort B – Expansion Expanded subject enrollment at final selected dose B ~12 MuSK-CAART IgG4-dominant disease Autoantibody titers drop after rituximab1,2 Pathogenic B cells incompletely eliminated by rituximab and persist during relapse3 Circulating anti-MuSK antibody titers may be ~90% lower than anti-DSG3 antibody in PV4,5,6 1 2 Study Endpoint & Objectives Primary Endpoint: Adverse Events, including DLT Secondary & Tertiary Objectives: CAART expansion/persistence, effect on serum anti-MuSK antibody titer, use of concomitant systemic medications, effect on clinical symptoms, manufacturing success rate Compelling biologic rationale, similar to PV Differentiated market opportunity Total U.S. MG prevalence 50,000 to 80,000 patients MuSK+ MG comprises 6-7.5% of total MG population Compared to AChR+ MG, MuSK+ disease is typically more severe with limited treatment options MuSK+ disease has early onset, 7:1 females * 500M refers to the number of MuSK-CAART cells infused for patients in dosing cohort A1 (M – millions). Hain, Berit, et al. "Successful treatment of MuSK antibody–positive MG with rituximab." Muscle & Nerve. 33.4 (2006): 575-580. Illa, Isabel, et al. "Sustained response to Rituximab in anti-AChR and anti-MuSK positive Myasthenia Gravis patients." Journal of neuroimmunology 201 (2008): 90-94. Jiang, Ruoyi, et al. "Single-cell repertoire tracing identifies rituximab-resistant B cells during myasthenia gravis relapses." JCI insight 5.14 (2020). Matthews, Ian, et al. "Muscle-specific receptor tyrosine kinase autoantibodies—a new immunoprecipitation assay." Clinica chimica acta 348.1-2 (2004): 95-99. McConville, John, et al. "Detection and characterization of MuSK antibodies in seronegative myasthenia gravis." Annals of neurology 55.4 (2004): 580-584. Marino, Mariapaola, et al. "Long-lasting rituximab-induced reduction of specific—but not total—IgG4 in MuSK-positive myasthenia gravis." Frontiers in immunology 11 (2020): 613. A total of 6 subjects will need to have received the final selected dose in Part A of the study. Cohorts A3 and A4 will be enrolled concurrently after cohort A2 is well-tolerated with a preference for enrollment into A4. Fast Track Designation Orphan Drug Designation

Corporate Summary
Three-stage approach allows for efficient allocation of capital while leveraging experienced partners Manufacturing strategy CDMOs shown are currently contracted for select CAART product candidates. Stage 3: Commercialization & Scale-Up Data-gated, staged investment Stage 1: Penn Stage 2: CDMOs & CABA Process Cell processing capacity secured through Penn partnership SOPs previously used to develop multiple clinical stage CAR T products Clinical vector validated CDMOs for vector and cell processing with commercial support capabilities1 Leasing followed by engineering and build out of Cabaletta-owned manufacturing facility, and/or Establishment of a strategic partnership to rapidly & reliably scale manufacturing, leveraging the partner’s manufacturing expertise 2021 – 2019 –

BOARD OF DIRECTORS Cabaletta Bio leadership LEADERSHIP TEAM Anup Marda Chief Financial Officer Arun Das, M.D. Chief Business Officer David J. Chang, M.D., M.P.H., FACR Chief Medical Officer Martha O’Connor Chief HR Officer Michael Gerard General Counsel Steven Nichtberger, M.D. President, CEO & Chairman Heather Harte-Hall Chief Compliance Officer Samik Basu, M.D. Chief Scientific Officer Gwendolyn Binder, Ph.D. President, Science & Technology SCIENTIFIC ADVISORY BOARD Track record of operational success employing novel cell therapies in autoimmunity across preclinical, clinical, manufacturing & regulatory domains Aimee Payne, M.D., Ph.D. Co-Founder and Co-Chair Michael C. Milone, M.D., Ph.D. Co-Founder and Co-Chair Carl June, M.D. Jay Siegel, M.D. Brian Daniels, M.D. Steven Nichtberger, M.D. Richard Henriques, M.B.A. Scott Brun, M.D. Mark Simon, M.B.A. Catherine Bollard, M.D. Drew Weissman, M.D., Ph.D. Iain McInnes, Ph.D., FRCP, FRSE, FMedSci Georg Schett, M.D.
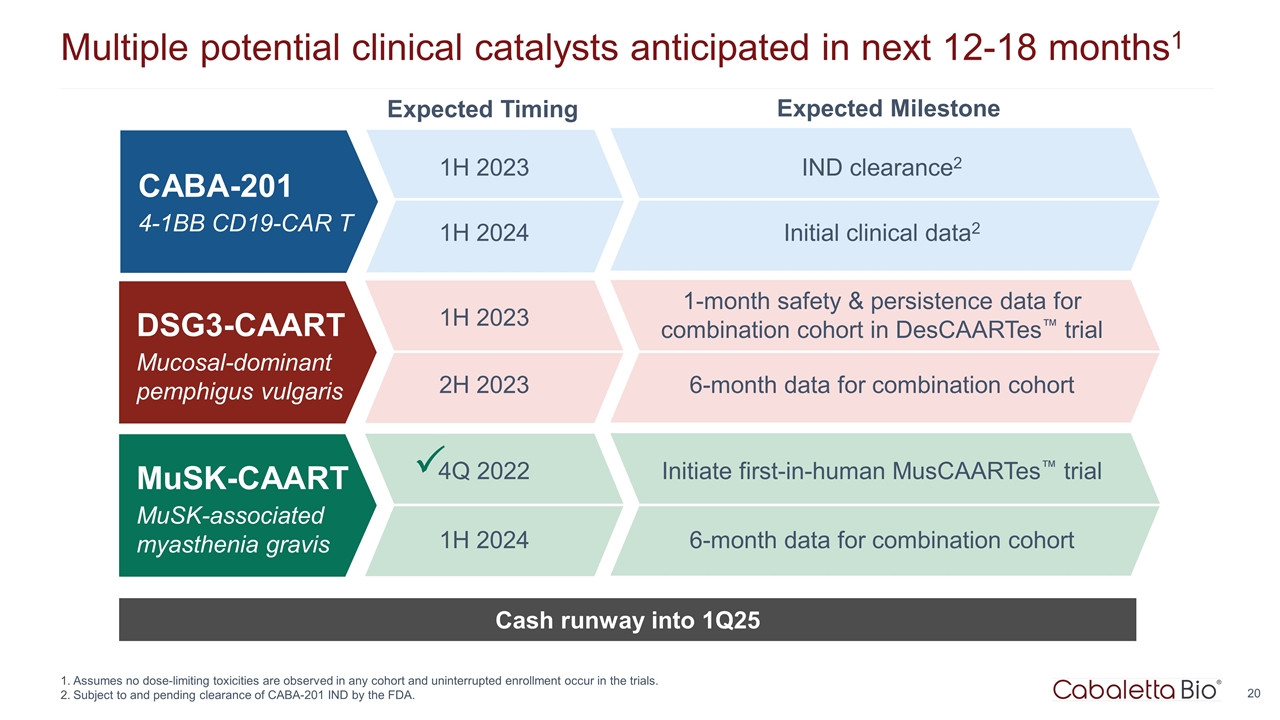
Multiple potential clinical catalysts anticipated in next 12-18 months1 Assumes no dose-limiting toxicities are observed in any cohort and uninterrupted enrollment occur in the trials. Subject to and pending clearance of CABA-201 IND by the FDA. Cash runway into 1Q25 CABA-201 4-1BB CD19-CAR T Expected Timing Expected Milestone 1H 2023 IND clearance2 1H 2024 Initial clinical data2 DSG3-CAART Mucosal-dominant pemphigus vulgaris 1H 2023 1-month safety & persistence data for combination cohort in DesCAARTes™ trial 2H 2023 6-month data for combination cohort MuSK-CAART MuSK-associated myasthenia gravis 4Q 2022 Initiate first-in-human MusCAARTes™ trial 1H 2024 6-month data for combination cohort P

Corporate Presentation MARCH 2023