

Corporate Presentation may 2021 Exhibit 99.2

Disclaimer The following presentation, including any printed or electronic copy of these slides, the talks given by the presenters, the information communicated during any delivery of the presentation and any question and answer session and any document or material distributed at or in connection with the presentation (collectively, the “Presentation”) has been prepared by Cabaletta Bio, Inc. (“we,” “us,” “our,” “Cabaletta” or the “Company”) and is made for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy securities, nor shall there be any sale of any securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. This Presentation does not purport to be a prospectus, to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this Presentation unless stated otherwise, and neither this Presentation, nor any sale of securities, shall under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This Presentation may contain “forward-looking statements” relating to our business, operations, and financial conditions, including, but not limited to, current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our preclinical and clinical results and other future conditions. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Factors which could cause actual results to differ materially from those in the forward-looking statements include, among others, the success, cost, and timing of our product candidate development activities and preclinical studies and clinical trials, our ability to obtain and maintain regulatory approval for our product candidates, our ability to commercialize our product candidates, future agreements with third parties in connection with the development or commercialization of our product candidates, the size and growth potential of the market for our product candidates, our ability to contract with third-party suppliers and manufacturers and our ability to develop internal manufacturing capabilities and facilities, the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing, and our ability to obtain and maintain intellectual property protection for our product candidates. Various risks, uncertainties and assumptions could cause actual results to differ materially from those anticipated or implied in our forward-looking statements. Such risks and uncertainties include, but are not limited to, uncertainties caused by adverse economic conditions, including, without limitation, as a result of extraordinary events or circumstances such as the COVID-19 pandemic, and any business interruptions to our operations or to those of our clinical sites, manufacturers, suppliers, or other vendors resulting from the COVID-19 pandemic or similar public health crisis. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ materially from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our 2020 annual report on Form 10-K, as well as discussions of potential risks, uncertainties, and other important factors in our other filings with the Securities and Exchange Commission. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. The Company is the owner of various trademarks, trade names and service marks. Certain other trademarks, trade names and service marks appearing in this Presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this Presentation are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.

Develop and launch the first curative targeted cellular therapies for patients with autoimmune diseases

Cabaletta overview Developing highly specific CAAR T products to treat B cell-mediated autoimmune diseases Where there is a biologic opportunity for deep and durable, perhaps curative, responses Leveraging a clinically validated CAR T product design and manufacturing process through Penn alliance Preclinical pipeline led by MuSK-CAART for myasthenia gravis - IND filing planned in 2H21 PLA2R-CAART pre-IND meeting with FDA anticipated in 2H21 – ~75% of 1⁰ membranous nephropathy patients have PLA2R antibodies Product portfolio2 currently targeting diseases that affect over 80,000 patients in the US Issued U.S. patent on lead clinical program with emerging differentiated IP portfolio First issued CAAR T product patent covers all or any part of the relevant human antigens (DSG3 and DSG1) Phase 1 DesCAARTesTM trial ongoing for patients with mucosal pemphigus vulgaris (mPV) No DLTs or any clinically relevant toxicities observed in the 1st 8 days following infusion for all 3 patients in the 1st cohort DSG3-CAART cells were detected at low levels via qPCR in both patients evaluated to date 20M cell dose without lymphodepletion and in the presence of circulating anti-DSG3 antibodies within patients Target engagement data from cohort 1, and acute safety data from the 1st 3 cohorts anticipated this year1 Cash runway through at least 4Q22 with $102M in cash and investments as of March 31, 2021 Assumes no dose-limiting toxicities are observed during cohort and uninterrupted enrollment occurs in the trial. Includes five disclosed product candidates and two undisclosed pipeline disease targets in our pipeline through expansion of our Sponsored Research Agreement with the University of Pennsylvania.
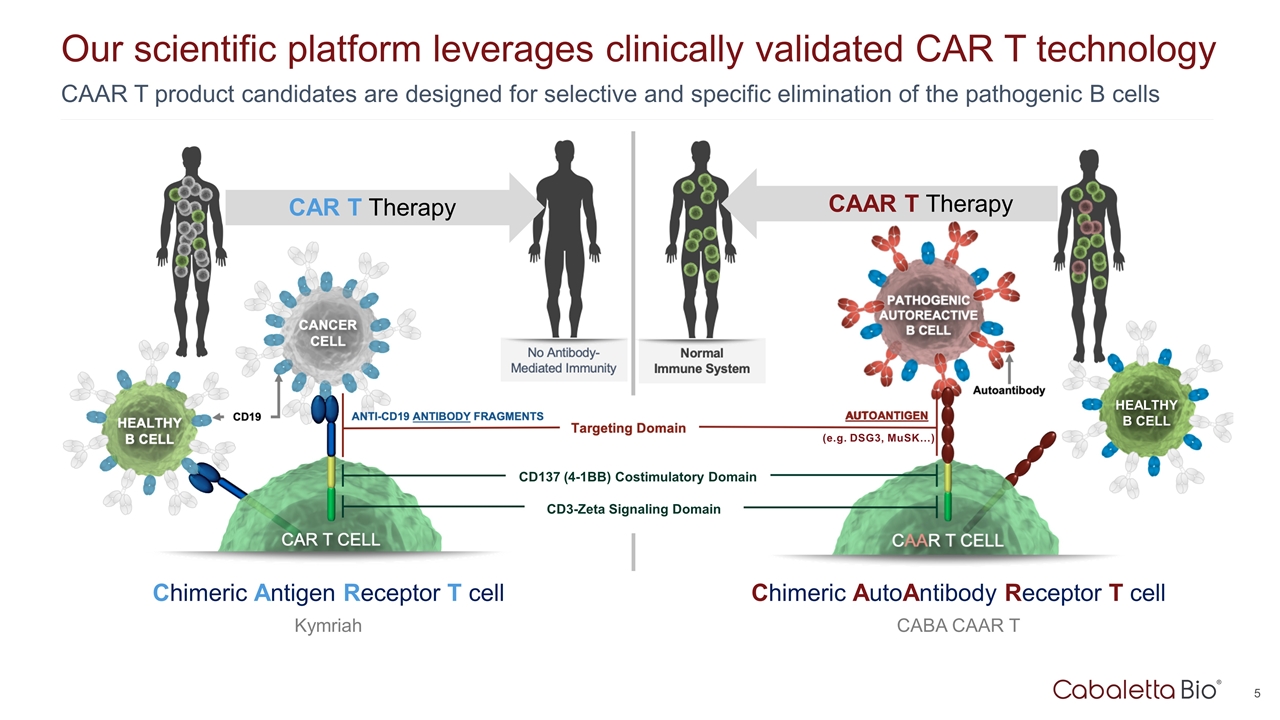
Our scientific platform leverages clinically validated CAR T technology CAR T Therapy Chimeric Antigen Receptor T cell Kymriah CAAR T product candidates are designed for selective and specific elimination of the pathogenic B cells CAAR T Therapy Chimeric AutoAntibody Receptor T cell CABA CAAR T AUTOANTIGEN (e.g. DSG3, MuSK…) CD137 (4-1BB) Costimulatory Domain CD3-Zeta Signaling Domain HEALTHY B CELL
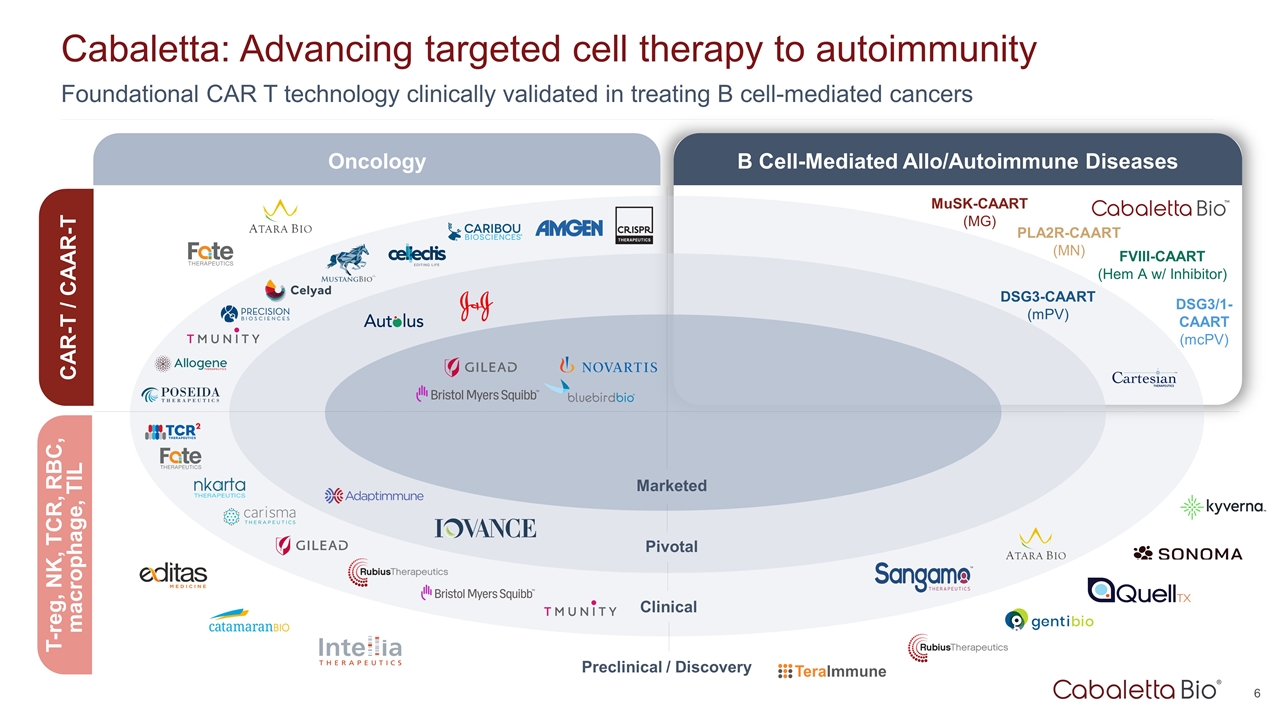
Foundational CAR T technology clinically validated in treating B cell-mediated cancers Cabaletta: Advancing targeted cell therapy to autoimmunity Marketed Pivotal Clinical Preclinical / Discovery DSG3-CAART (mPV) MuSK-CAART (MG) FVIII-CAART (Hem A w/ Inhibitor) DSG3/1-CAART (mcPV) Oncology B Cell-Mediated Allo/Autoimmune Diseases CAR-T / CAAR-T T-reg, NK, TCR, RBC, macrophage, TIL PLA2R-CAART (MN)
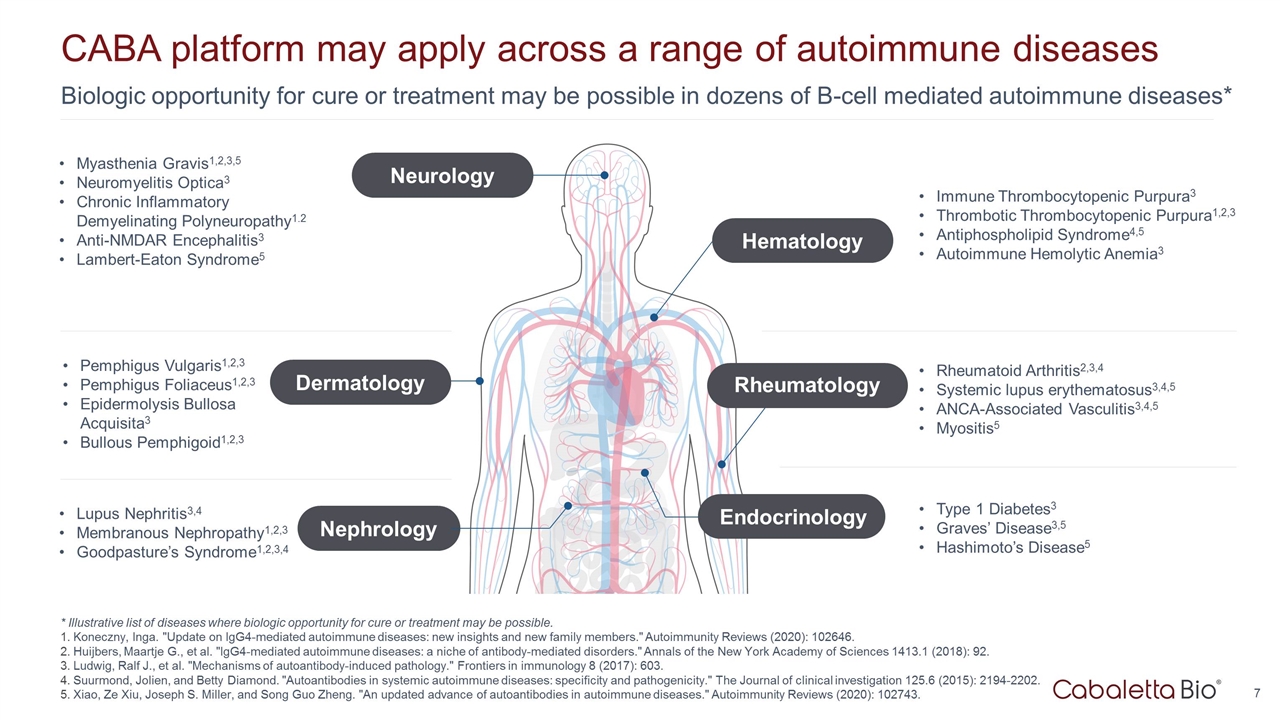
Biologic opportunity for cure or treatment may be possible in dozens of B-cell mediated autoimmune diseases* CABA platform may apply across a range of autoimmune diseases Pemphigus Vulgaris1,2,3 Pemphigus Foliaceus1,2,3 Epidermolysis Bullosa Acquisita3 Bullous Pemphigoid1,2,3 Lupus Nephritis3,4 Membranous Nephropathy1,2,3 Goodpasture’s Syndrome1,2,3,4 Myasthenia Gravis1,2,3,5 Neuromyelitis Optica3 Chronic Inflammatory Demyelinating Polyneuropathy1.2 Anti-NMDAR Encephalitis3 Lambert-Eaton Syndrome5 Immune Thrombocytopenic Purpura3 Thrombotic Thrombocytopenic Purpura1,2,3 Antiphospholipid Syndrome4,5 Autoimmune Hemolytic Anemia3 Rheumatoid Arthritis2,3,4 Systemic lupus erythematosus3,4,5 ANCA-Associated Vasculitis3,4,5 Myositis5 Type 1 Diabetes3 Graves’ Disease3,5 Hashimoto’s Disease5 * Illustrative list of diseases where biologic opportunity for cure or treatment may be possible. Koneczny, Inga. "Update on IgG4-mediated autoimmune diseases: new insights and new family members." Autoimmunity Reviews (2020): 102646. Huijbers, Maartje G., et al. "IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders." Annals of the New York Academy of Sciences 1413.1 (2018): 92. Ludwig, Ralf J., et al. "Mechanisms of autoantibody-induced pathology." Frontiers in immunology 8 (2017): 603. Suurmond, Jolien, and Betty Diamond. "Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity." The Journal of clinical investigation 125.6 (2015): 2194-2202. Xiao, Ze Xiu, Joseph S. Miller, and Song Guo Zheng. "An updated advance of autoantibodies in autoimmune diseases." Autoimmunity Reviews (2020): 102743. Dermatology Nephrology Neurology Hematology Rheumatology Endocrinology
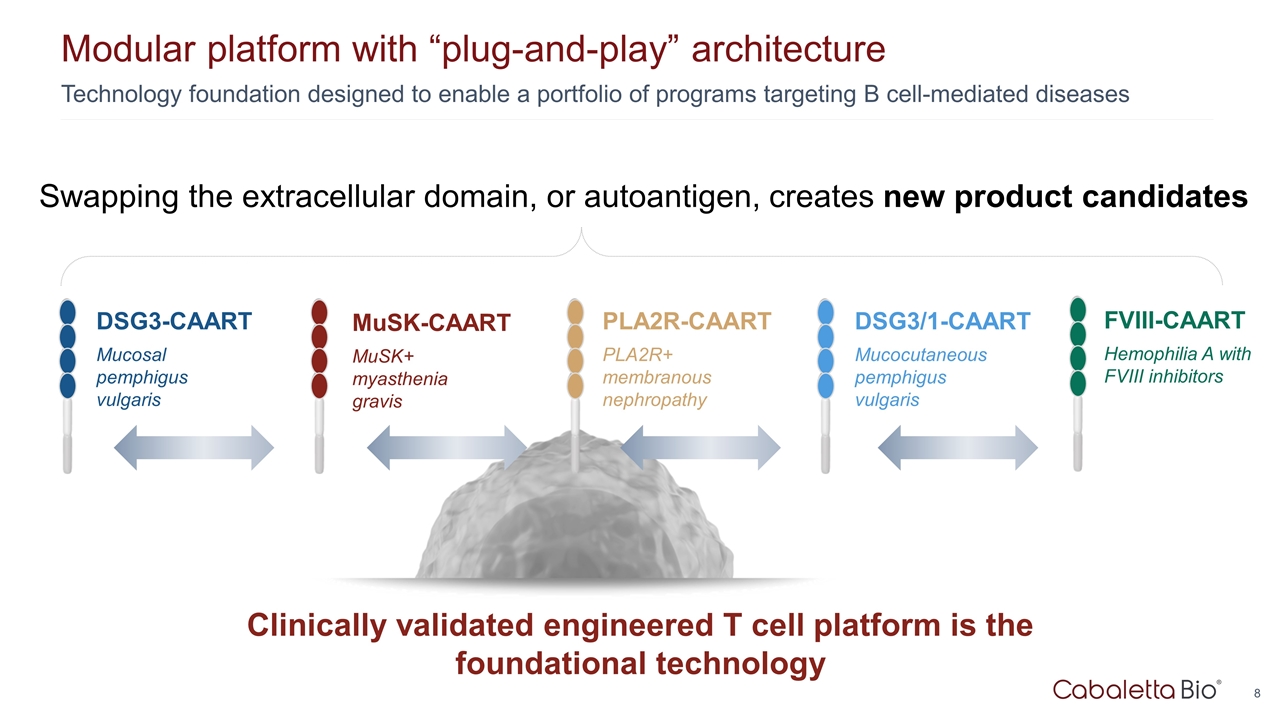
Technology foundation designed to enable a portfolio of programs targeting B cell-mediated diseases Modular platform with “plug-and-play” architecture Clinically validated engineered T cell platform is the foundational technology PLA2R-CAART PLA2R+ membranous nephropathy DSG3-CAART Mucosal pemphigus vulgaris MuSK-CAART MuSK+ myasthenia gravis DSG3/1-CAART Mucocutaneous pemphigus vulgaris Swapping the extracellular domain, or autoantigen, creates new product candidates FVIII-CAART Hemophilia A with FVIII inhibitors
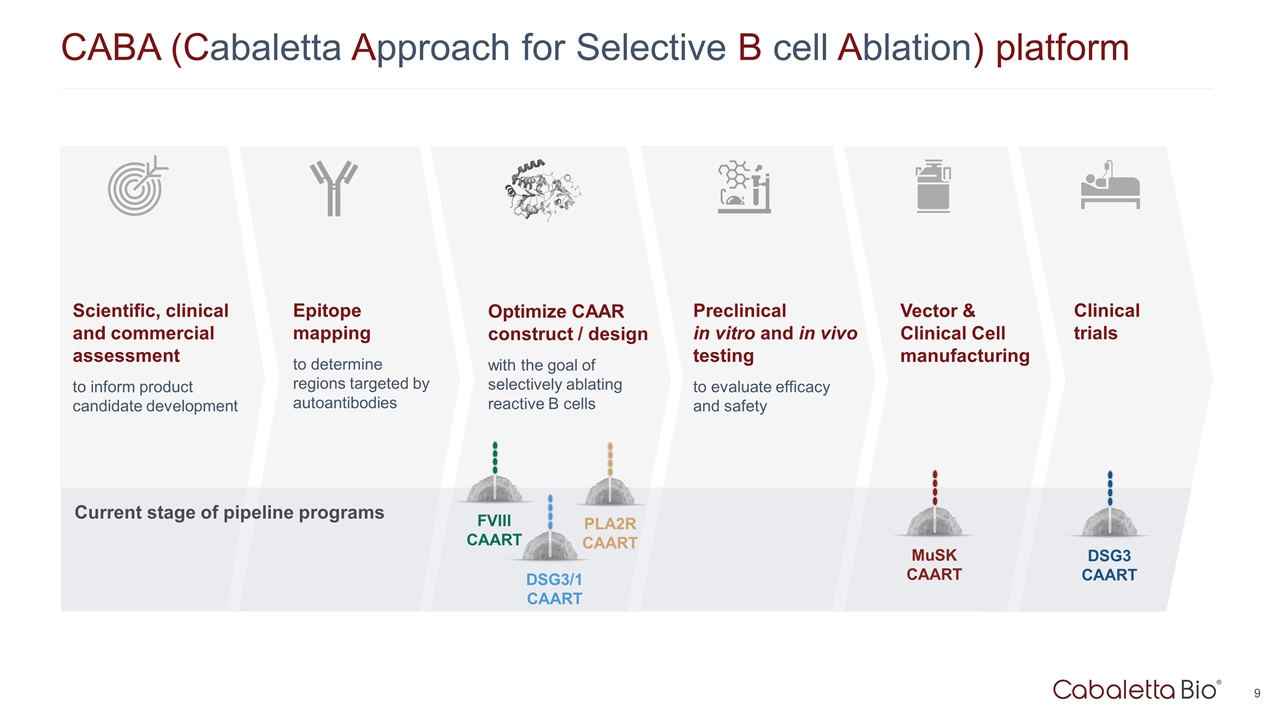
Epitope mapping to determine regions targeted by autoantibodies Optimize CAAR construct / design with the goal of selectively ablating reactive B cells Preclinical in vitro and in vivo testing to evaluate efficacy and safety Scientific, clinical and commercial assessment to inform product candidate development Vector & Clinical Cell manufacturing Clinical trials CABA (Cabaletta Approach for Selective B cell Ablation) platform DSG3 CAART MuSK CAART PLA2R CAART FVIII CAART DSG3/1 CAART Current stage of pipeline programs
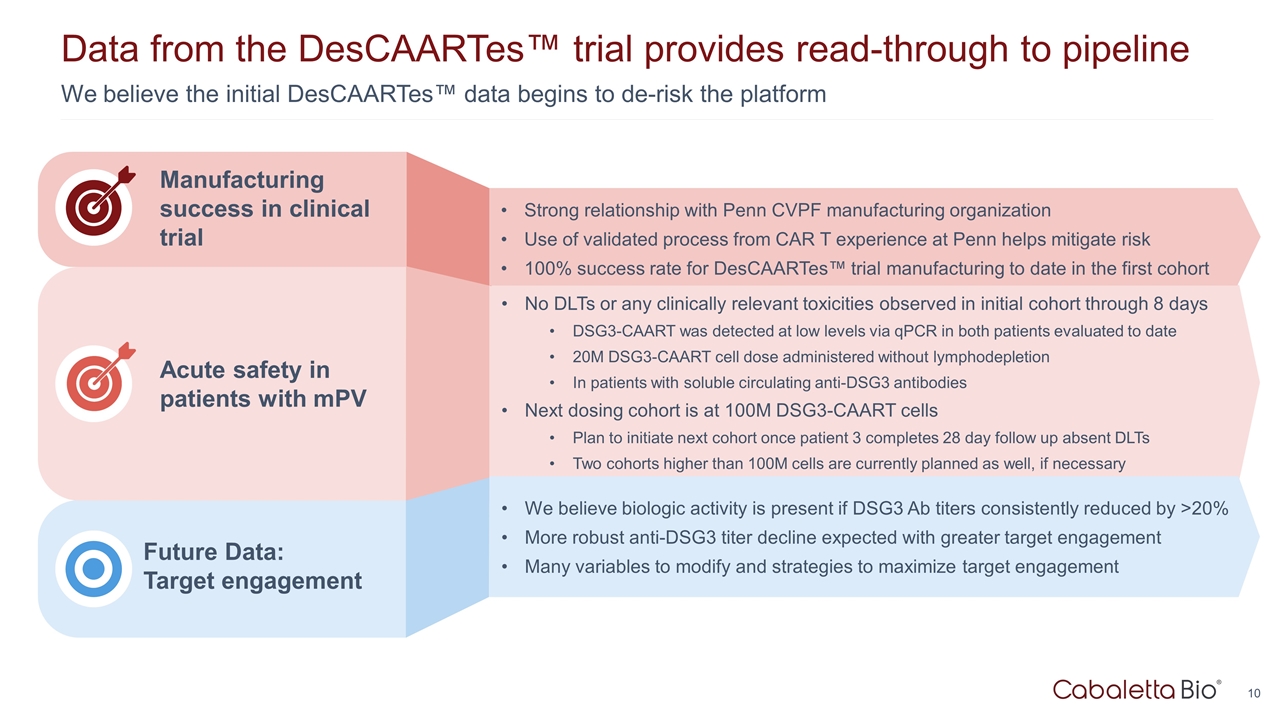
We believe the initial DesCAARTes™ data begins to de-risk the platform Data from the DesCAARTes™ trial provides read-through to pipeline Acute safety in patients with mPV Future Data: Target engagement Manufacturing success in clinical trial Strong relationship with Penn CVPF manufacturing organization Use of validated process from CAR T experience at Penn helps mitigate risk 100% success rate for DesCAARTes™ trial manufacturing to date in the first cohort No DLTs or any clinically relevant toxicities observed in initial cohort through 8 days DSG3-CAART was detected at low levels via qPCR in both patients evaluated to date 20M DSG3-CAART cell dose administered without lymphodepletion In patients with soluble circulating anti-DSG3 antibodies Next dosing cohort is at 100M DSG3-CAART cells Plan to initiate next cohort once patient 3 completes 28 day follow up absent DLTs Two cohorts higher than 100M cells are currently planned as well, if necessary We believe biologic activity is present if DSG3 Ab titers consistently reduced by >20% More robust anti-DSG3 titer decline expected with greater target engagement Many variables to modify and strategies to maximize target engagement
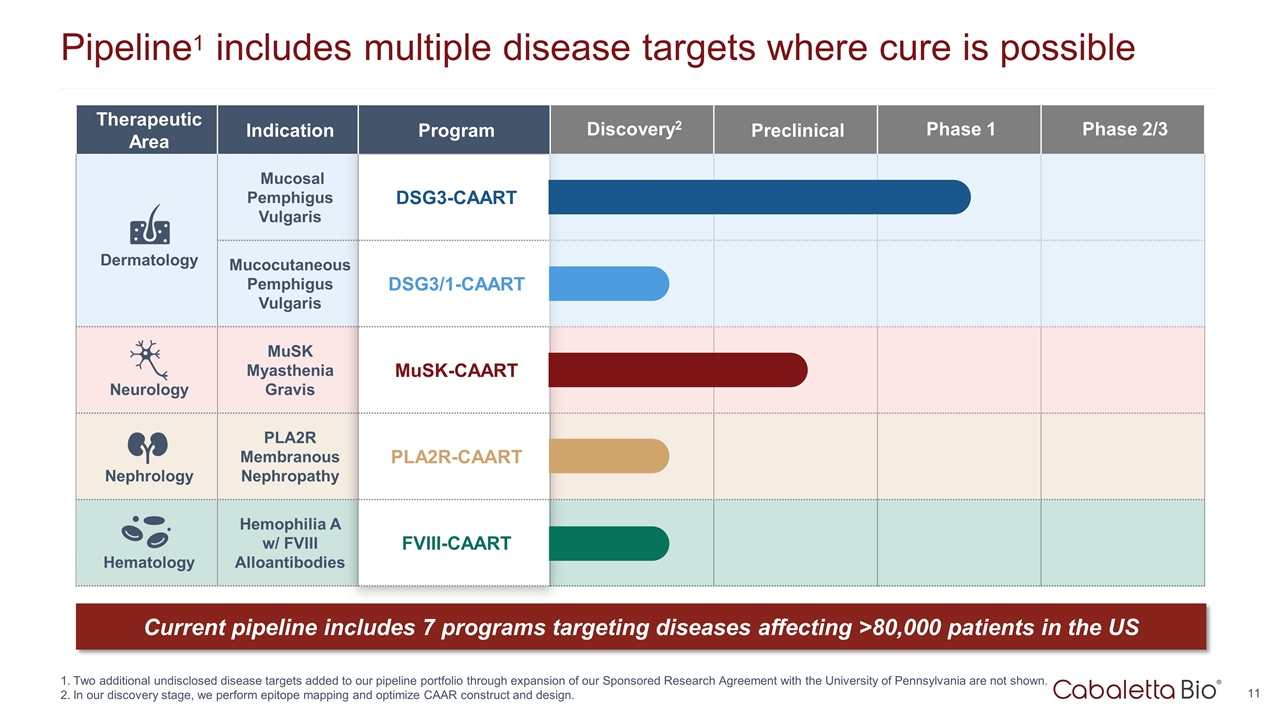
Therapeutic Area Indication Program Discovery2 Preclinical Phase 1 Phase 2/3 Dermatology Mucosal Pemphigus Vulgaris DSG3-CAART Mucocutaneous Pemphigus Vulgaris DSG3/1-CAART Neurology MuSK Myasthenia Gravis MuSK-CAART Nephrology PLA2R Membranous Nephropathy PLA2R-CAART Hematology Hemophilia A w/ FVIII Alloantibodies FVIII-CAART Pipeline1 includes multiple disease targets where cure is possible Two additional undisclosed disease targets added to our pipeline portfolio through expansion of our Sponsored Research Agreement with the University of Pennsylvania are not shown. In our discovery stage, we perform epitope mapping and optimize CAAR construct and design. Current pipeline includes 7 programs targeting diseases affecting >80,000 patients in the US

DSG3-CAART for patients with mucosal pemphigus vulgaris
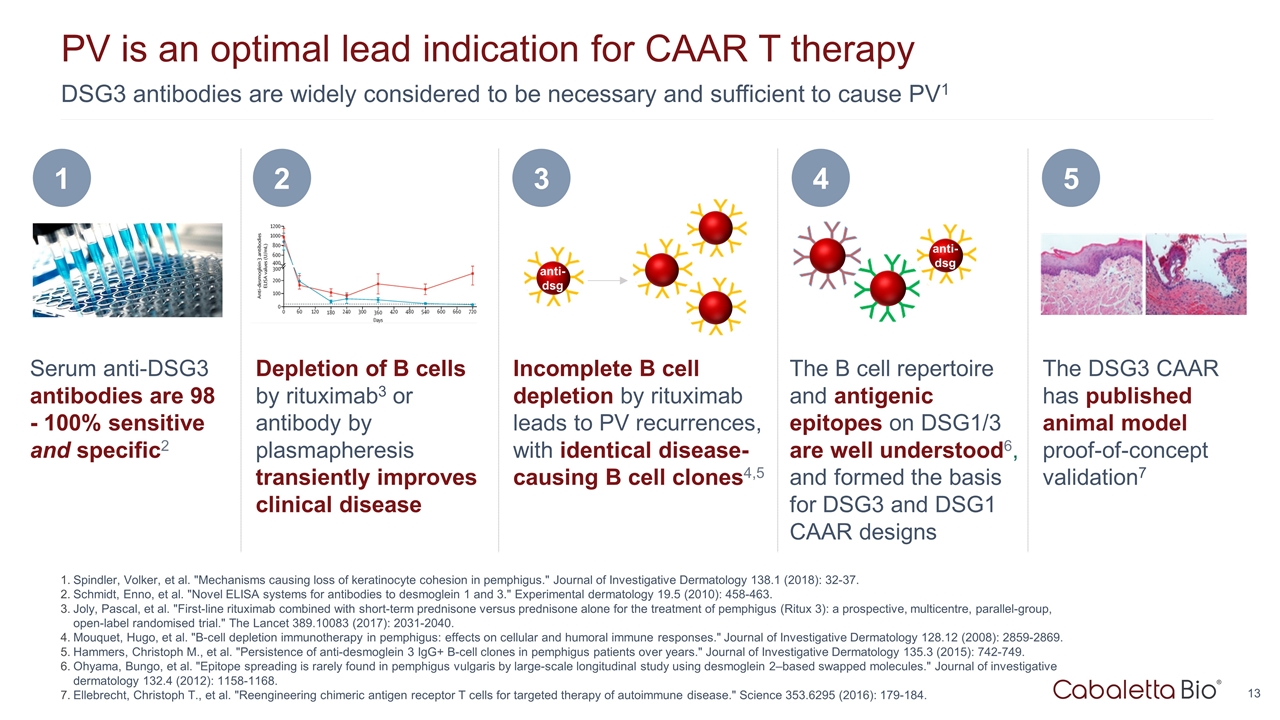
DSG3 antibodies are widely considered to be necessary and sufficient to cause PV1 PV is an optimal lead indication for CAAR T therapy Spindler, Volker, et al. "Mechanisms causing loss of keratinocyte cohesion in pemphigus." Journal of Investigative Dermatology 138.1 (2018): 32-37. Schmidt, Enno, et al. "Novel ELISA systems for antibodies to desmoglein 1 and 3." Experimental dermatology 19.5 (2010): 458-463. Joly, Pascal, et al. "First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial." The Lancet 389.10083 (2017): 2031-2040. Mouquet, Hugo, et al. "B-cell depletion immunotherapy in pemphigus: effects on cellular and humoral immune responses." Journal of Investigative Dermatology 128.12 (2008): 2859-2869. Hammers, Christoph M., et al. "Persistence of anti-desmoglein 3 IgG+ B-cell clones in pemphigus patients over years." Journal of Investigative Dermatology 135.3 (2015): 742-749. Ohyama, Bungo, et al. "Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudinal study using desmoglein 2–based swapped molecules." Journal of investigative dermatology 132.4 (2012): 1158-1168. Ellebrecht, Christoph T., et al. "Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease." Science 353.6295 (2016): 179-184. Serum anti-DSG3 antibodies are 98 - 100% sensitive and specific2 1 The DSG3 CAAR has published animal model proof-of-concept validation7 5 Depletion of B cells by rituximab3 or antibody by plasmapheresis transiently improves clinical disease 2 The B cell repertoire and antigenic epitopes on DSG1/3 are well understood6, and formed the basis for DSG3 and DSG1 CAAR designs 4 Incomplete B cell depletion by rituximab leads to PV recurrences, with identical disease-causing B cell clones4,5 3 anti- dsg anti- dsg
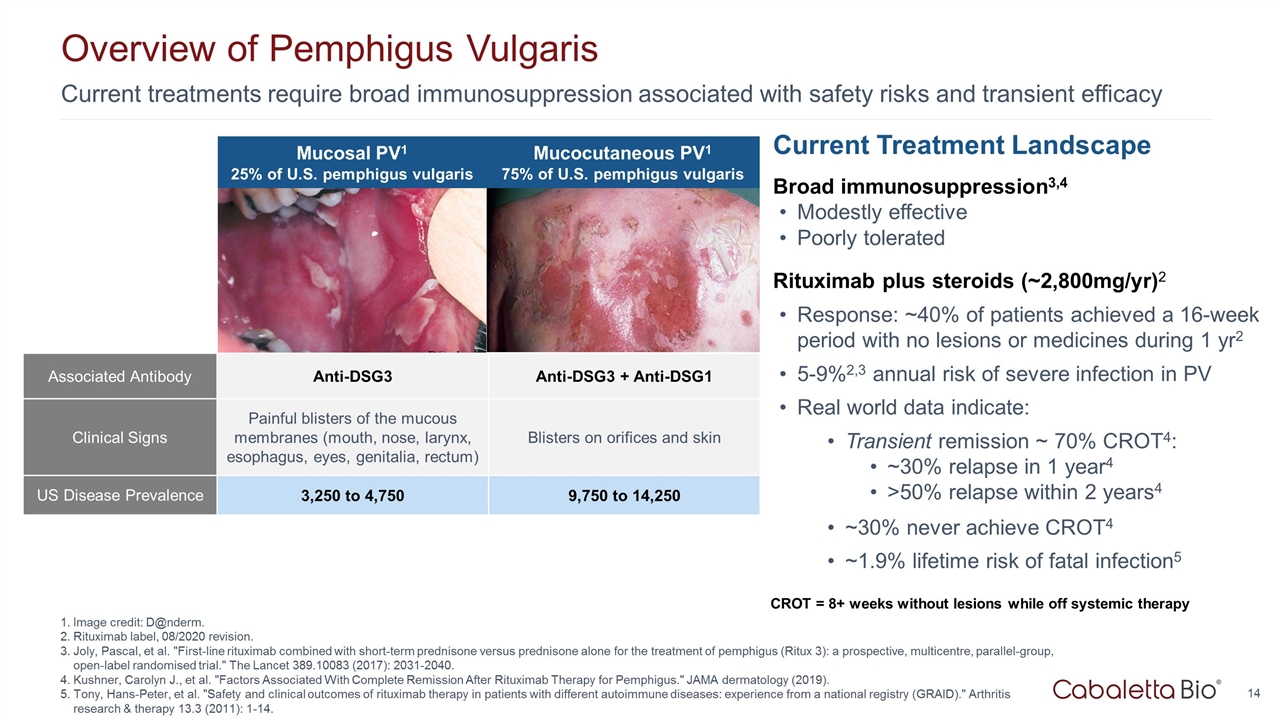
Current treatments require broad immunosuppression associated with safety risks and transient efficacy Overview of Pemphigus Vulgaris Image credit: D@nderm. Rituximab label, 08/2020 revision. Joly, Pascal, et al. "First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial." The Lancet 389.10083 (2017): 2031-2040. Kushner, Carolyn J., et al. "Factors Associated With Complete Remission After Rituximab Therapy for Pemphigus." JAMA dermatology (2019). Tony, Hans-Peter, et al. "Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID)." Arthritis research & therapy 13.3 (2011): 1-14. Current Treatment Landscape Broad immunosuppression3,4 Modestly effective Poorly tolerated Rituximab plus steroids (~2,800mg/yr)2 Response: ~40% of patients achieved a 16-week period with no lesions or medicines during 1 yr2 5-9%2,3 annual risk of severe infection in PV Real world data indicate: Transient remission ~ 70% CROT4: ~30% relapse in 1 year4 >50% relapse within 2 years4 ~30% never achieve CROT4 ~1.9% lifetime risk of fatal infection5 CROT = 8+ weeks without lesions while off systemic therapy http://www.danderm-pdv.is.kkh.dk/atlas/3-157.html http://www.dermis.net/bilder/CD008/550px/img0042.jpg Mucosal PV1 25% of U.S. pemphigus vulgaris Mucocutaneous PV1 75% of U.S. pemphigus vulgaris Associated Antibody Anti-DSG3 Anti-DSG3 + Anti-DSG1 Clinical Signs Painful blisters of the mucous membranes (mouth, nose, larynx, esophagus, eyes, genitalia, rectum) Blisters on orifices and skin US Disease Prevalence 3,250 to 4,750 9,750 to 14,250
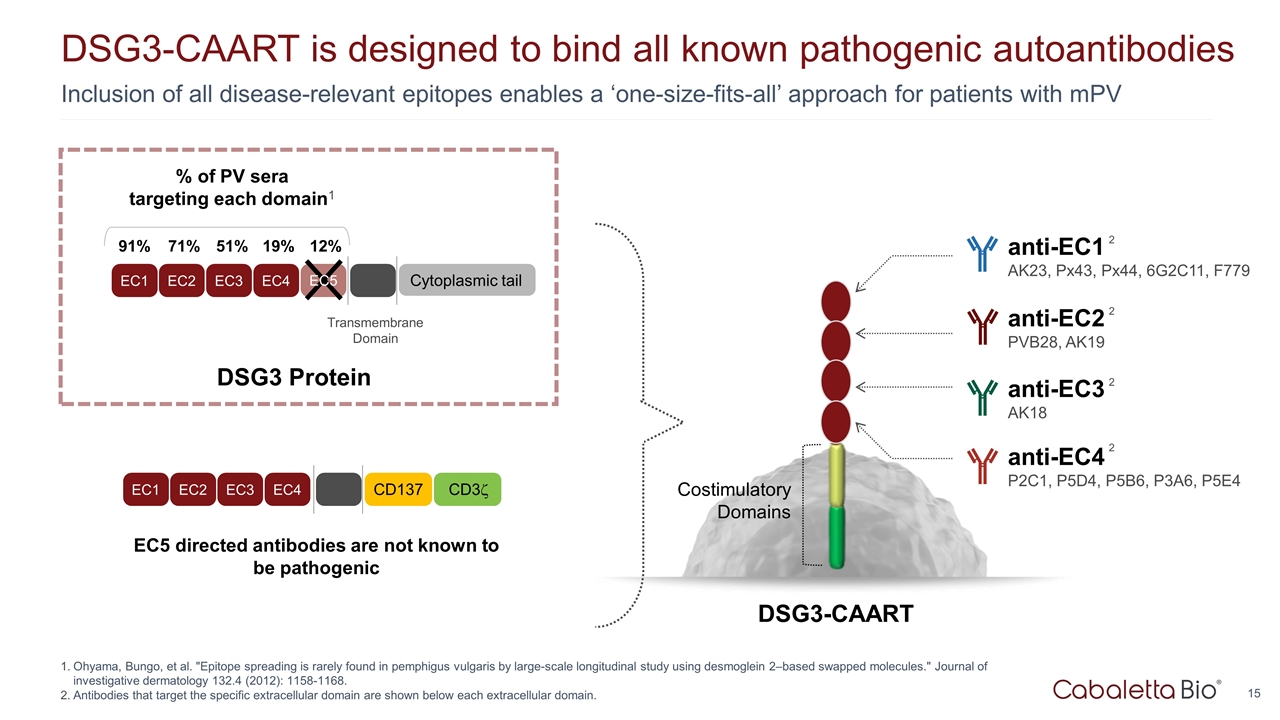
Inclusion of all disease-relevant epitopes enables a ‘one-size-fits-all’ approach for patients with mPV DSG3-CAART is designed to bind all known pathogenic autoantibodies Ohyama, Bungo, et al. "Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudinal study using desmoglein 2–based swapped molecules." Journal of investigative dermatology 132.4 (2012): 1158-1168. Antibodies that target the specific extracellular domain are shown below each extracellular domain. EC5 directed antibodies are not known to be pathogenic DSG3-CAART Costimulatory Domains anti-EC4 P2C1, P5D4, P5B6, P3A6, P5E4 2 anti-EC3 AK18 2 anti-EC2 PVB28, AK19 2 anti-EC1 AK23, Px43, Px44, 6G2C11, F779 2 EC1 EC2 EC3 EC4 Transmembrane Domain Cytoplasmic tail % of PV sera targeting each domain1 91% 71% 51% 19% 12% EC5 EC1 EC2 EC3 EC4 CD137 CD3z DSG3 Protein
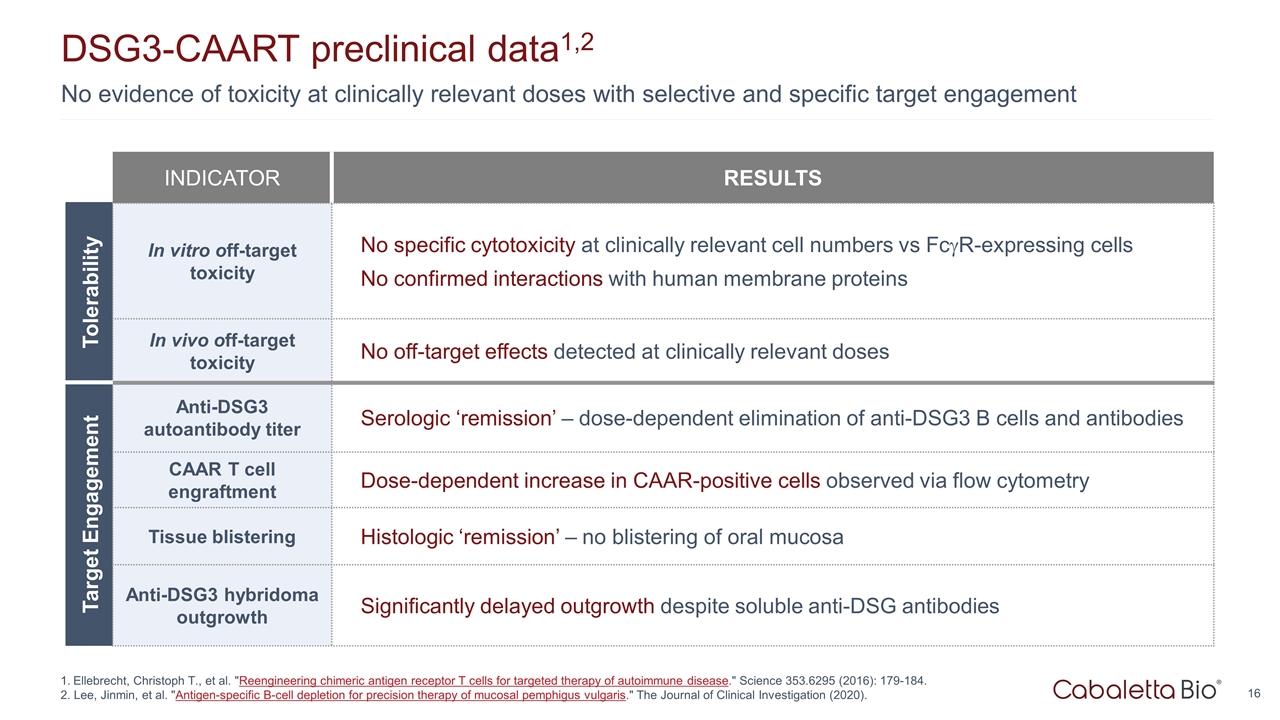
Tolerability Target Engagement No evidence of toxicity at clinically relevant doses with selective and specific target engagement DSG3-CAART preclinical data1,2 INDICATOR RESULTS In vitro off-target toxicity No specific cytotoxicity at clinically relevant cell numbers vs FcgR-expressing cells No confirmed interactions with human membrane proteins In vivo off-target toxicity No off-target effects detected at clinically relevant doses Anti-DSG3 autoantibody titer Serologic ‘remission’ – dose-dependent elimination of anti-DSG3 B cells and antibodies CAAR T cell engraftment Dose-dependent increase in CAAR-positive cells observed via flow cytometry Tissue blistering Histologic ‘remission’ – no blistering of oral mucosa Anti-DSG3 hybridoma outgrowth Significantly delayed outgrowth despite soluble anti-DSG antibodies Ellebrecht, Christoph T., et al. "Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease." Science 353.6295 (2016): 179-184. 2. Lee, Jinmin, et al. "Antigen-specific B-cell depletion for precision therapy of mucosal pemphigus vulgaris." The Journal of Clinical Investigation (2020).
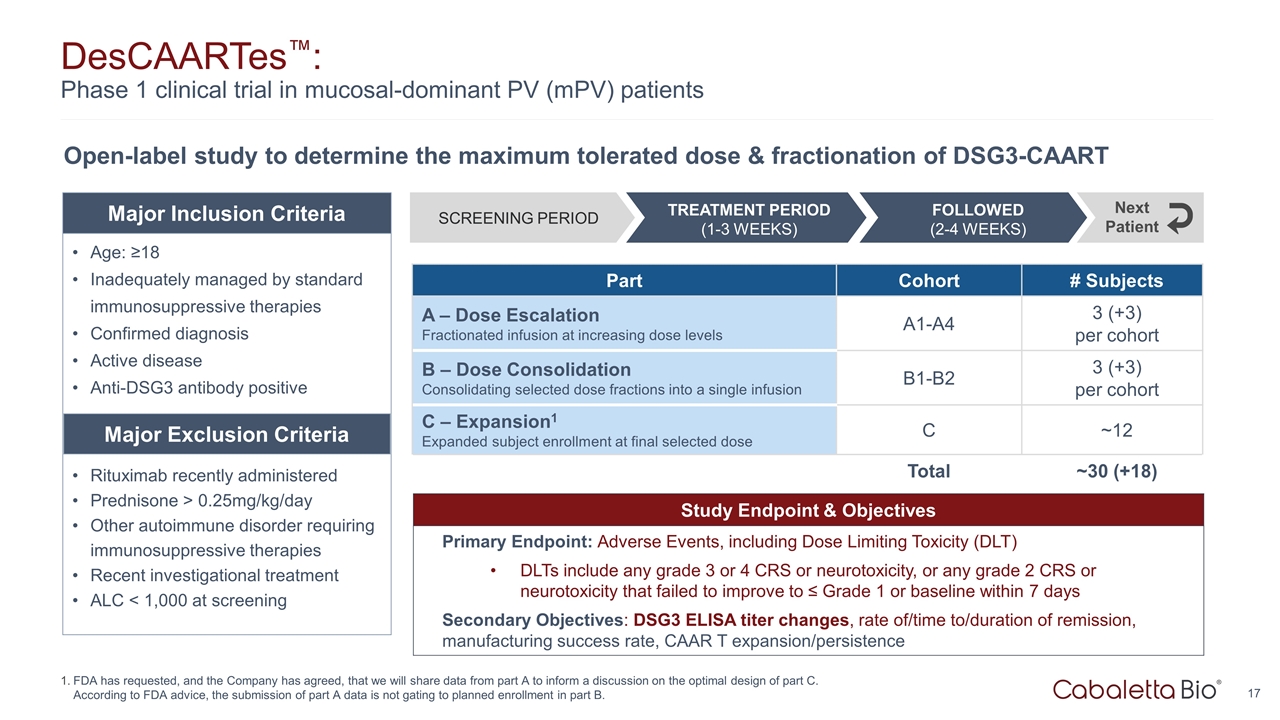
Study Endpoint & Objectives Primary Endpoint: Adverse Events, including Dose Limiting Toxicity (DLT) DLTs include any grade 3 or 4 CRS or neurotoxicity, or any grade 2 CRS or neurotoxicity that failed to improve to ≤ Grade 1 or baseline within 7 days Secondary Objectives: DSG3 ELISA titer changes, rate of/time to/duration of remission, manufacturing success rate, CAAR T expansion/persistence DesCAARTes™: Phase 1 clinical trial in mucosal-dominant PV (mPV) patients FDA has requested, and the Company has agreed, that we will share data from part A to inform a discussion on the optimal design of part C. According to FDA advice, the submission of part A data is not gating to planned enrollment in part B. Open-label study to determine the maximum tolerated dose & fractionation of DSG3-CAART Part Cohort # Subjects A – Dose Escalation Fractionated infusion at increasing dose levels A1-A4 3 (+3) per cohort B – Dose Consolidation Consolidating selected dose fractions into a single infusion B1-B2 3 (+3) per cohort C – Expansion1 Expanded subject enrollment at final selected dose C ~12 Total ~30 (+18) Age: ≥18 Inadequately managed by standard immunosuppressive therapies Confirmed diagnosis Active disease Anti-DSG3 antibody positive Rituximab recently administered Prednisone > 0.25mg/kg/day Other autoimmune disorder requiring immunosuppressive therapies Recent investigational treatment ALC < 1,000 at screening Major Inclusion Criteria Major Exclusion Criteria SCREENING PERIOD FOLLOWED (2-4 WEEKS) TREATMENT PERIOD (1-3 WEEKS) Next Patient
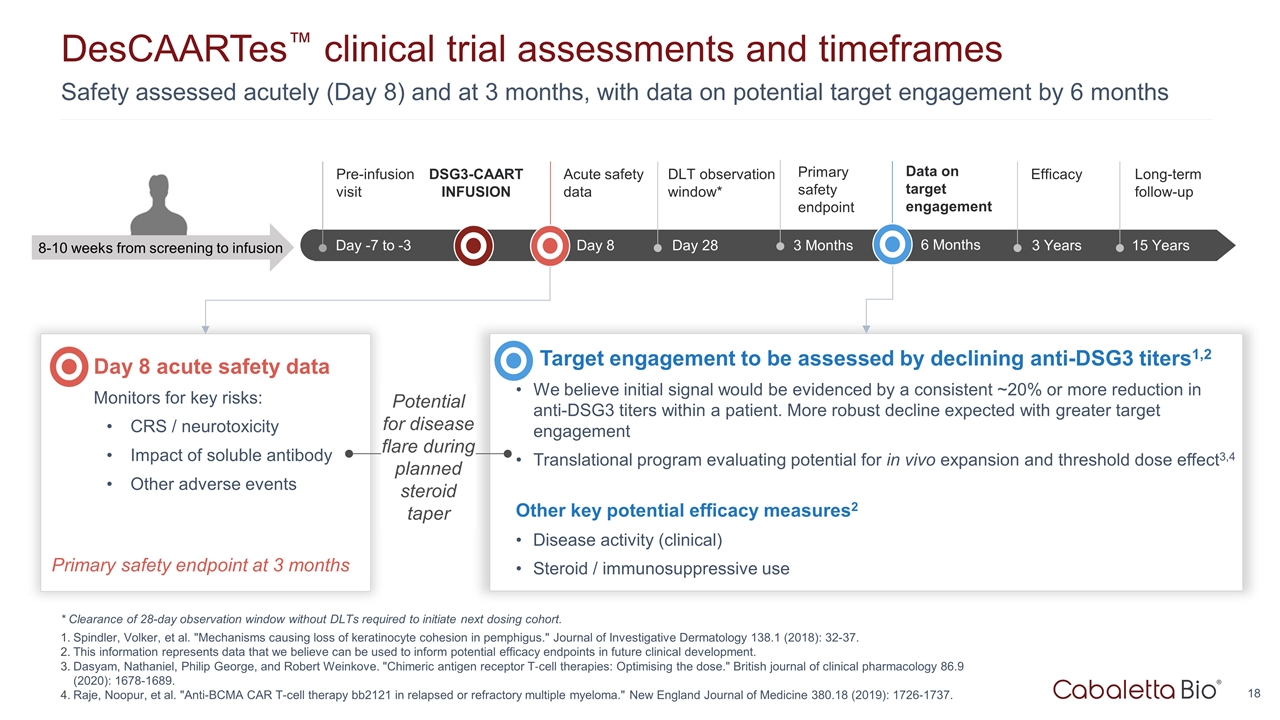
Safety assessed acutely (Day 8) and at 3 months, with data on potential target engagement by 6 months DesCAARTes™ clinical trial assessments and timeframes * Clearance of 28-day observation window without DLTs required to initiate next dosing cohort. Spindler, Volker, et al. "Mechanisms causing loss of keratinocyte cohesion in pemphigus." Journal of Investigative Dermatology 138.1 (2018): 32-37. This information represents data that we believe can be used to inform potential efficacy endpoints in future clinical development. Dasyam, Nathaniel, Philip George, and Robert Weinkove. "Chimeric antigen receptor T‐cell therapies: Optimising the dose." British journal of clinical pharmacology 86.9 (2020): 1678-1689. Raje, Noopur, et al. "Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma." New England Journal of Medicine 380.18 (2019): 1726-1737. Up to Wk -18 Wk -18 to -1 Day -7 to -3 Pre-infusion visit 3 Years Efficacy 15 Years Long-term follow-up 3 Months Primary safety endpoint Day 8 Acute safety data 8-10 weeks from screening to infusion DSG3-CAART INFUSION Day 8 acute safety data Monitors for key risks: CRS / neurotoxicity Impact of soluble antibody Other adverse events Target engagement to be assessed by declining anti-DSG3 titers1,2 We believe initial signal would be evidenced by a consistent ~20% or more reduction in anti-DSG3 titers within a patient. More robust decline expected with greater target engagement Translational program evaluating potential for in vivo expansion and threshold dose effect3,4 Other key potential efficacy measures2 Disease activity (clinical) Steroid / immunosuppressive use Primary safety endpoint at 3 months Potential for disease flare during planned steroid taper Day 28 DLT observation window* 6 Months Data on target engagement
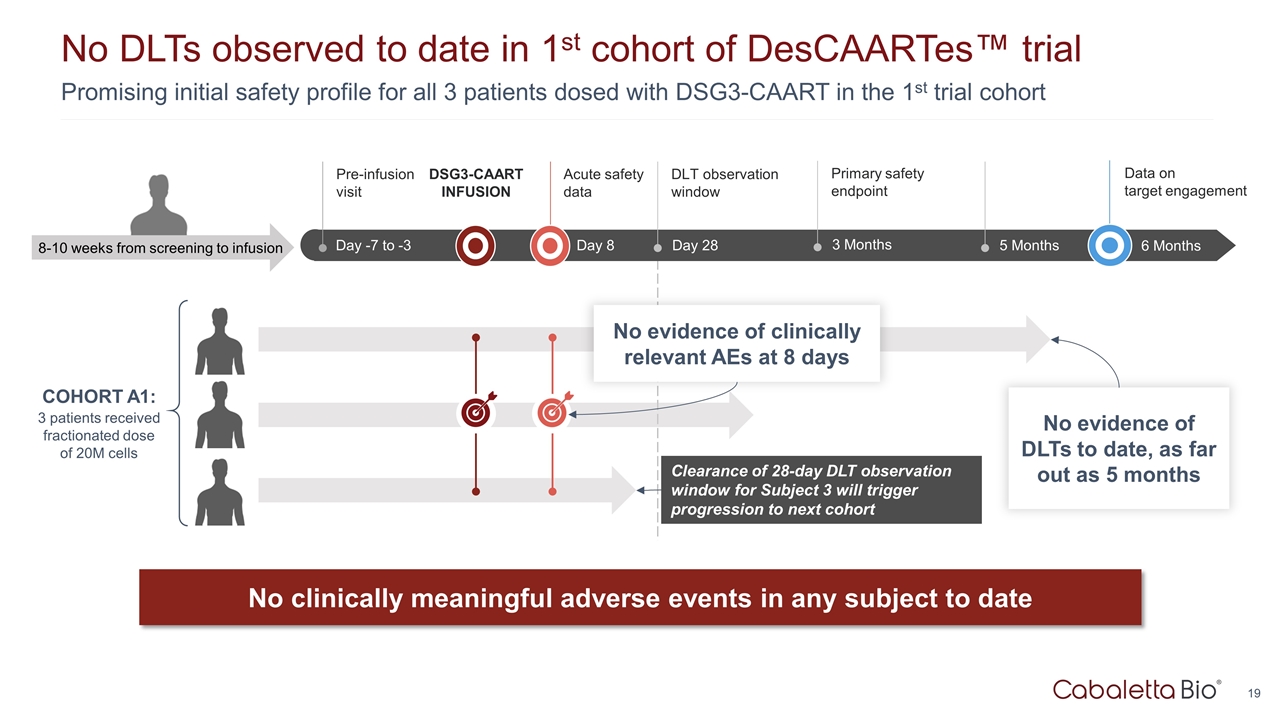
Promising initial safety profile for all 3 patients dosed with DSG3-CAART in the 1st trial cohort No DLTs observed to date in 1st cohort of DesCAARTes™ trial Up to Wk -18 Wk -18 to -1 Day -7 to -3 Pre-infusion visit 5 Months 6 Months Data on target engagement Day 8 Acute safety data 8-10 weeks from screening to infusion DSG3-CAART INFUSION Day 28 DLT observation window No clinically meaningful adverse events in any subject to date 3 Months Primary safety endpoint Clearance of 28-day DLT observation window for Subject 3 will trigger progression to next cohort No evidence of DLTs to date, as far out as 5 months No evidence of clinically relevant AEs at 8 days COHORT A1: 3 patients received fractionated dose of 20M cells
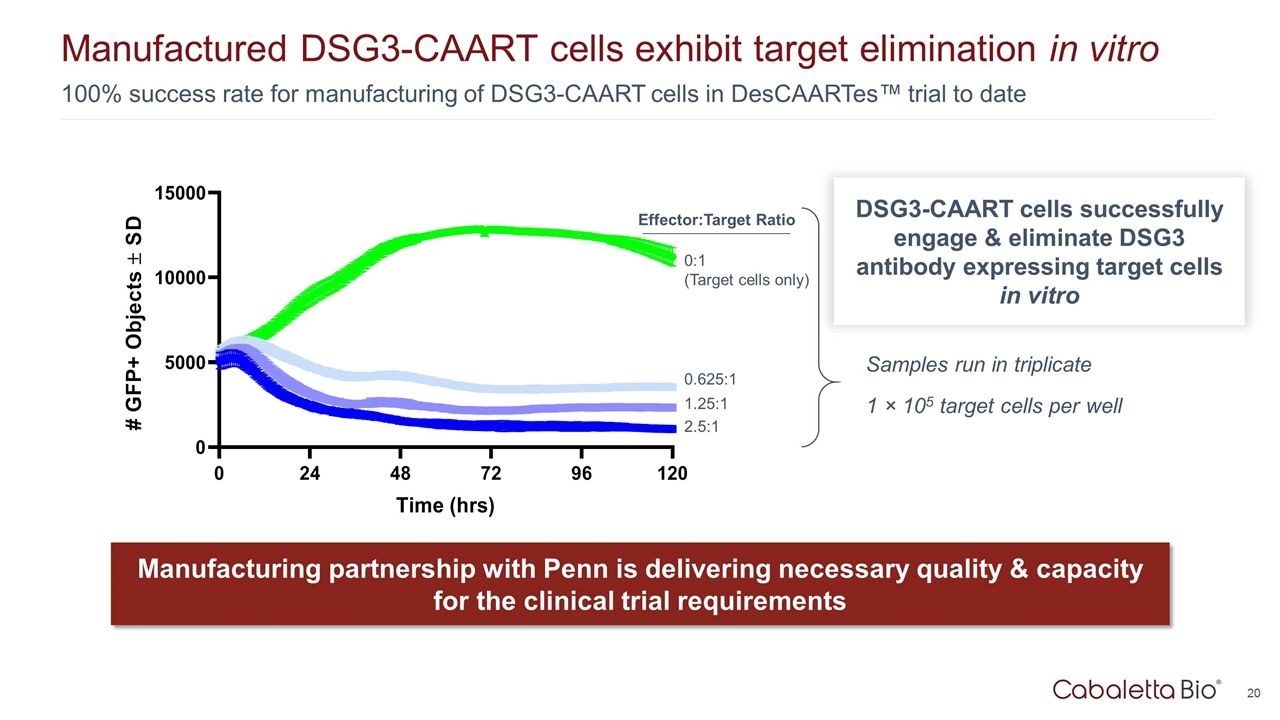
100% success rate for manufacturing of DSG3-CAART cells in DesCAARTes™ trial to date Manufactured DSG3-CAART cells exhibit target elimination in vitro Manufacturing partnership with Penn is delivering necessary quality & capacity for the clinical trial requirements 2.5:1 1.25:1 0.625:1 0:1 (Target cells only) Samples run in triplicate 1 × 105 target cells per well Effector:Target Ratio DSG3-CAART cells successfully engage & eliminate DSG3 antibody expressing target cells in vitro
No DLTs or clinically relevant toxicities in 1st three patients to date At a 20 million cell dose, in the absence of lymphodepletion Circulating anti-DSG3 antibodies present in all patients at infusion Patients 1 and 2 have completed the 28-day DLT monitoring period DSG3-CAART was observed at low levels via qPCR in both patients Patient 3 has completed the acute safety period (1st 8-days post-infusion) Evaluation for DSG3-CAART has not yet occurred Initial clinical safety profile in 1st cohort informed by several factors Future topline target engagement data to be disclosed on a cohort-by-cohort basis Target engagement in the 1st cohort possible, but not expected Topline target engagement data on 1st cohort to be reported in 2H21
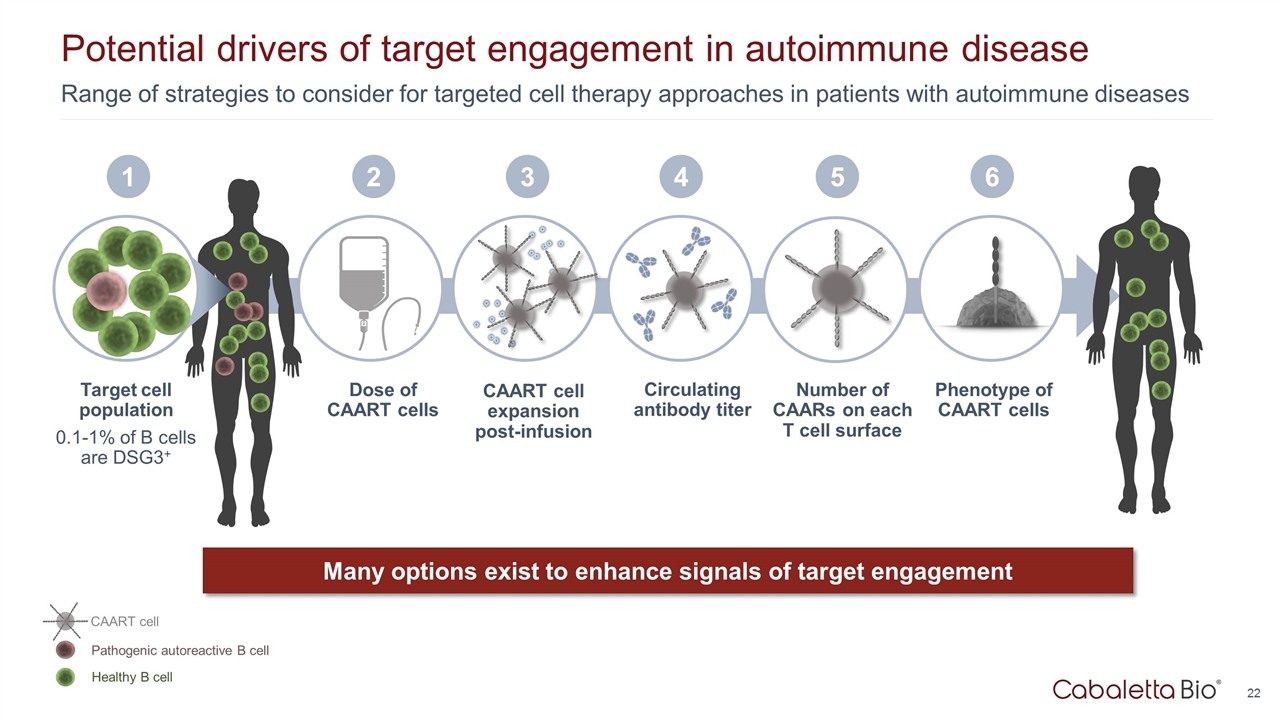
Dose of CAART cells 2 CAART cell expansion post-infusion 3 Circulating antibody titer 4 Range of strategies to consider for targeted cell therapy approaches in patients with autoimmune diseases Potential drivers of target engagement in autoimmune disease Target cell population 0.1-1% of B cells are DSG3+ Many options exist to enhance signals of target engagement 1 Phenotype of CAART cells 6 Healthy B cell Pathogenic autoreactive B cell CAART cell 5 Number of CAARs on each T cell surface
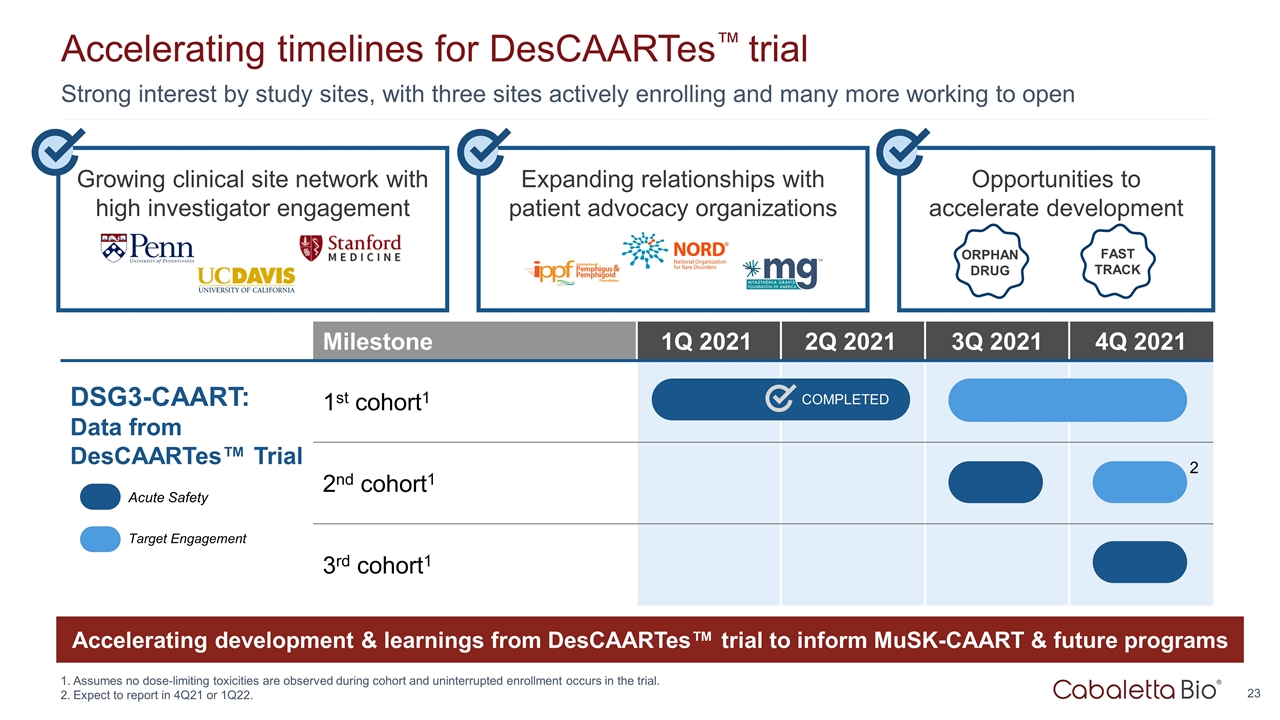
Expanding relationships with patient advocacy organizations Growing clinical site network with high investigator engagement Strong interest by study sites, with three sites actively enrolling and many more working to open Accelerating timelines for DesCAARTes™ trial Assumes no dose-limiting toxicities are observed during cohort and uninterrupted enrollment occurs in the trial. Expect to report in 4Q21 or 1Q22. Opportunities to accelerate development ORPHAN DRUG FAST TRACK Milestone 1Q 2021 2Q 2021 3Q 2021 4Q 2021 DSG3-CAART: Data from DesCAARTes™ Trial 1st cohort1 2nd cohort1 3rd cohort1 COMPLETED Acute Safety Target Engagement Accelerating development & learnings from DesCAARTes™ trial to inform MuSK-CAART & future programs 2
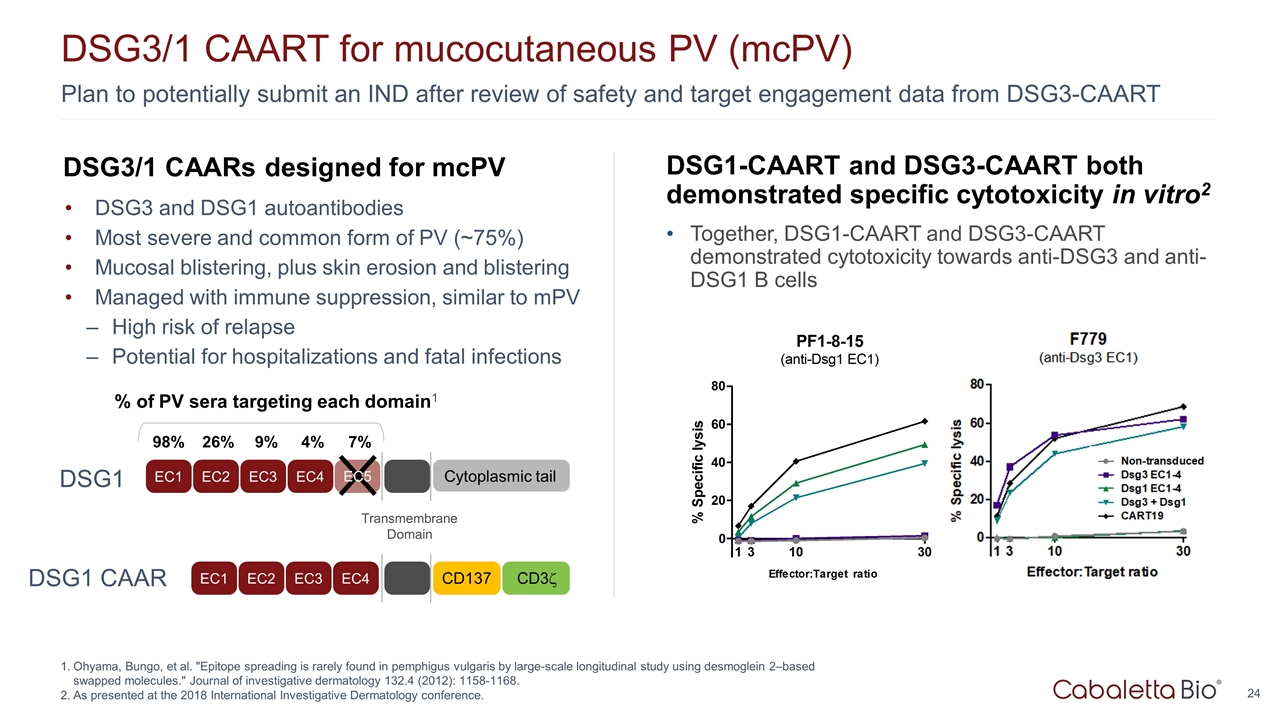
DSG3/1 CAARs designed for mcPV DSG3 and DSG1 autoantibodies Most severe and common form of PV (~75%) Mucosal blistering, plus skin erosion and blistering Managed with immune suppression, similar to mPV High risk of relapse Potential for hospitalizations and fatal infections Plan to potentially submit an IND after review of safety and target engagement data from DSG3-CAART DSG3/1 CAART for mucocutaneous PV (mcPV) Ohyama, Bungo, et al. "Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudinal study using desmoglein 2–based swapped molecules." Journal of investigative dermatology 132.4 (2012): 1158-1168. As presented at the 2018 International Investigative Dermatology conference. DSG1-CAART and DSG3-CAART both demonstrated specific cytotoxicity in vitro2 Together, DSG1-CAART and DSG3-CAART demonstrated cytotoxicity towards anti-DSG3 and anti-DSG1 B cells EC1 EC2 EC3 EC4 Transmembrane Domain Cytoplasmic tail % of PV sera targeting each domain1 98% 26% 9% 4% 7% EC5 EC1 EC2 EC3 EC4 CD137 CD3z DSG1 DSG1 CAAR

MuSK-CAART for patients with MuSK myasthenia gravis
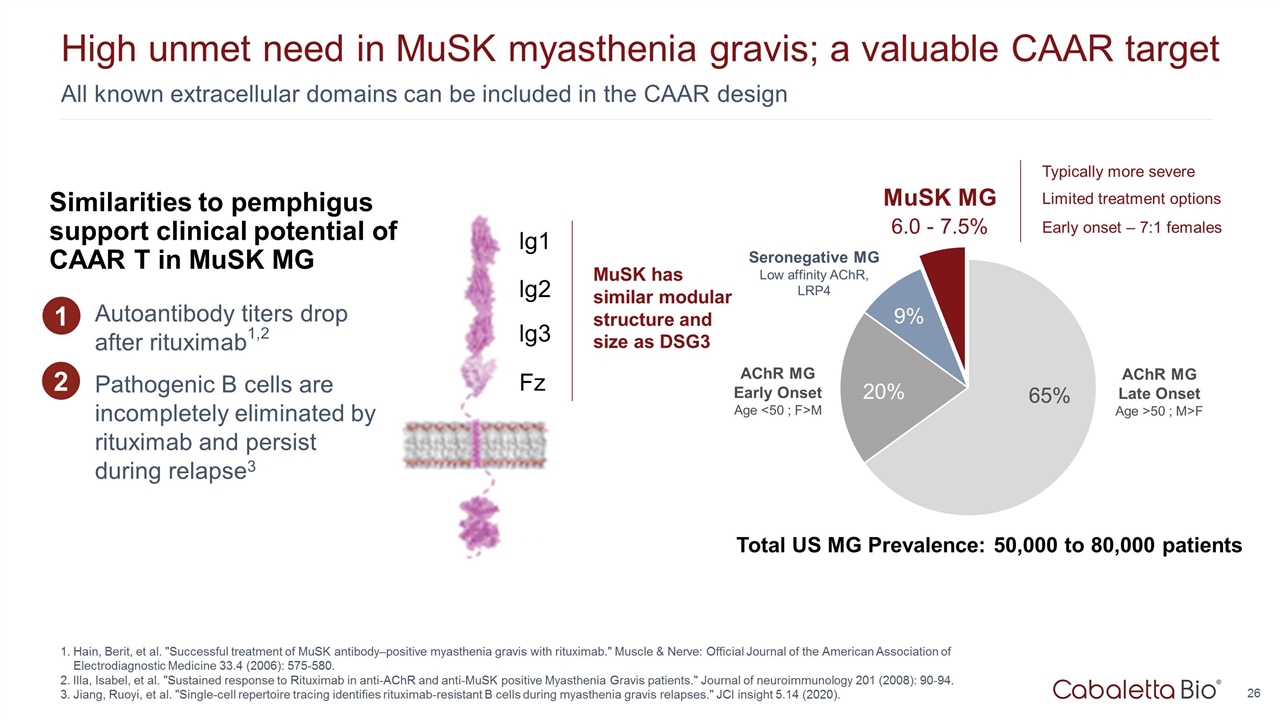
6.0 - 7.5% All known extracellular domains can be included in the CAAR design High unmet need in MuSK myasthenia gravis; a valuable CAAR target Hain, Berit, et al. "Successful treatment of MuSK antibody–positive myasthenia gravis with rituximab." Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 33.4 (2006): 575-580. Illa, Isabel, et al. "Sustained response to Rituximab in anti-AChR and anti-MuSK positive Myasthenia Gravis patients." Journal of neuroimmunology 201 (2008): 90-94. Jiang, Ruoyi, et al. "Single-cell repertoire tracing identifies rituximab-resistant B cells during myasthenia gravis relapses." JCI insight 5.14 (2020). AChR MG Early Onset Age <50 ; F>M AChR MG Late Onset Age >50 ; M>F MuSK MG Seronegative MG Low affinity AChR, LRP4 Total US MG Prevalence: 50,000 to 80,000 patients Typically more severe Limited treatment options Early onset – 7:1 females MuSK has similar modular structure and size as DSG3 lg1 lg2 lg3 Fz Autoantibody titers drop after rituximab1,2 Pathogenic B cells are incompletely eliminated by rituximab and persist during relapse3 1 Similarities to pemphigus support clinical potential of CAAR T in MuSK MG 2
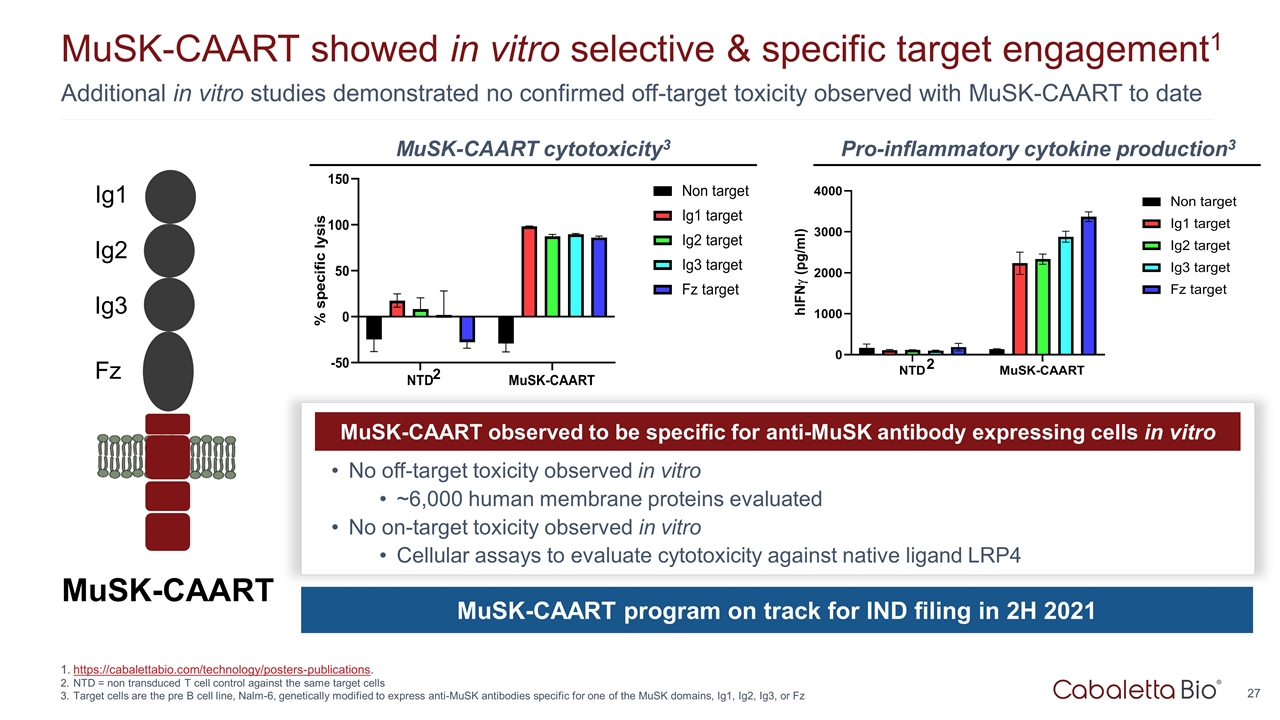
Additional in vitro studies demonstrated no confirmed off-target toxicity observed with MuSK-CAART to date MuSK-CAART showed in vitro selective & specific target engagement1 https://cabalettabio.com/technology/posters-publications. NTD = non transduced T cell control against the same target cells Target cells are the pre B cell line, Nalm-6, genetically modified to express anti-MuSK antibodies specific for one of the MuSK domains, Ig1, Ig2, Ig3, or Fz MuSK-CAART Ig1 Ig2 Ig3 Fz MuSK-CAART observed to be specific for anti-MuSK antibody expressing cells in vitro No off-target toxicity observed in vitro ~6,000 human membrane proteins evaluated No on-target toxicity observed in vitro Cellular assays to evaluate cytotoxicity against native ligand LRP4 MuSK-CAART program on track for IND filing in 2H 2021 MuSK-CAART cytotoxicity3 Pro-inflammatory cytokine production3 2 2
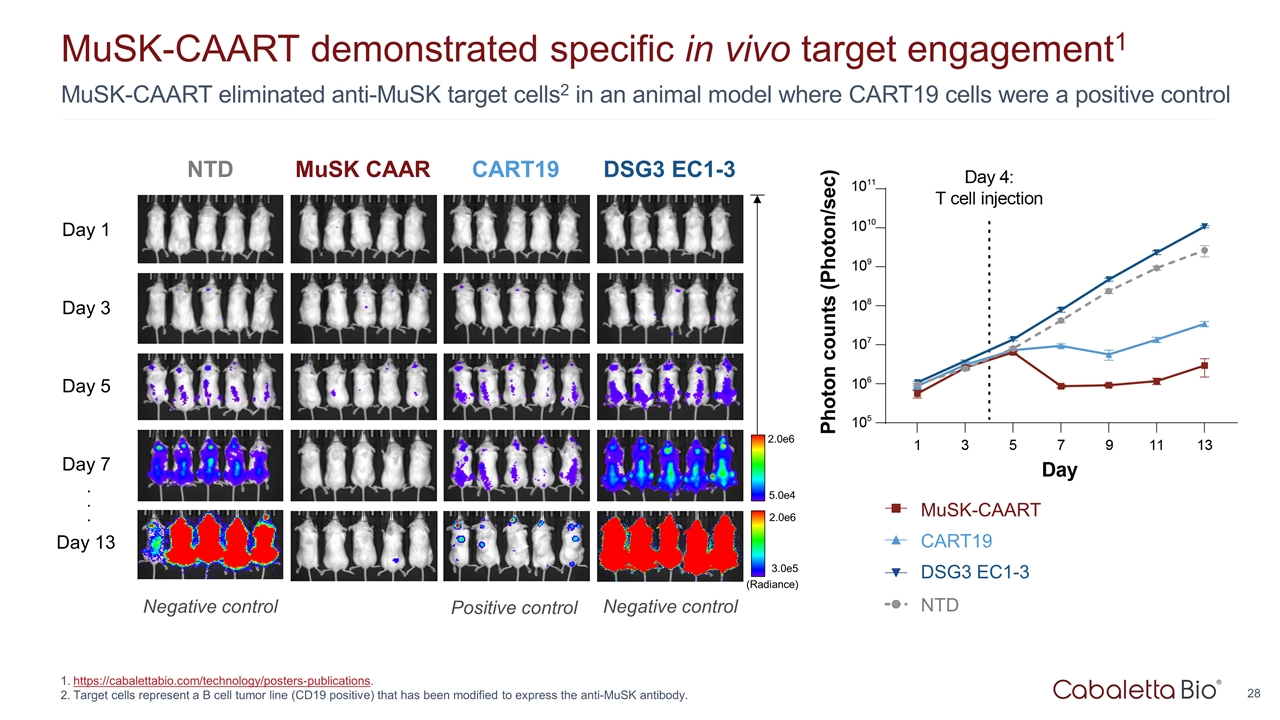
MuSK-CAART eliminated anti-MuSK target cells2 in an animal model where CART19 cells were a positive control MuSK-CAART demonstrated specific in vivo target engagement1 https://cabalettabio.com/technology/posters-publications. Target cells represent a B cell tumor line (CD19 positive) that has been modified to express the anti-MuSK antibody. MuSK CAAR CART19 DSG3 EC1-3 Day 1 Day 3 Day 5 Day 7 Day 13 5.0e4 2.0e6 (Radiance) 3.0e5 2.0e6 . . . NTD Positive control Negative control Negative control CART19 MuSK-CAART DSG3 EC1-3 NTD

PLA2R-CAART for patients with PLA2R-associated membranous nephropathy
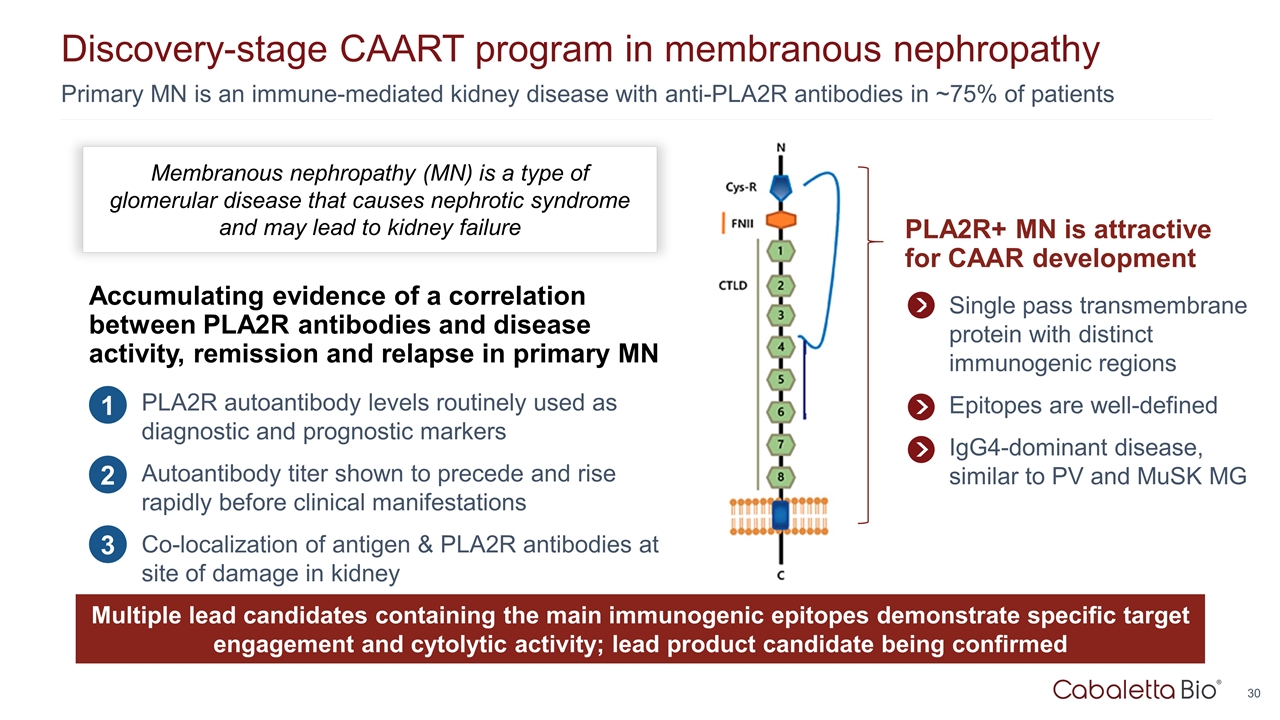
Primary MN is an immune-mediated kidney disease with anti-PLA2R antibodies in ~75% of patients Discovery-stage CAART program in membranous nephropathy PLA2R+ MN is attractive for CAAR development Single pass transmembrane protein with distinct immunogenic regions Epitopes are well-defined IgG4-dominant disease, similar to PV and MuSK MG Membranous nephropathy (MN) is a type of glomerular disease that causes nephrotic syndrome and may lead to kidney failure Accumulating evidence of a correlation between PLA2R antibodies and disease activity, remission and relapse in primary MN PLA2R autoantibody levels routinely used as diagnostic and prognostic markers Autoantibody titer shown to precede and rise rapidly before clinical manifestations Co-localization of antigen & PLA2R antibodies at site of damage in kidney 1 2 3 Multiple lead candidates containing the main immunogenic epitopes demonstrate specific target engagement and cytolytic activity; lead product candidate being confirmed
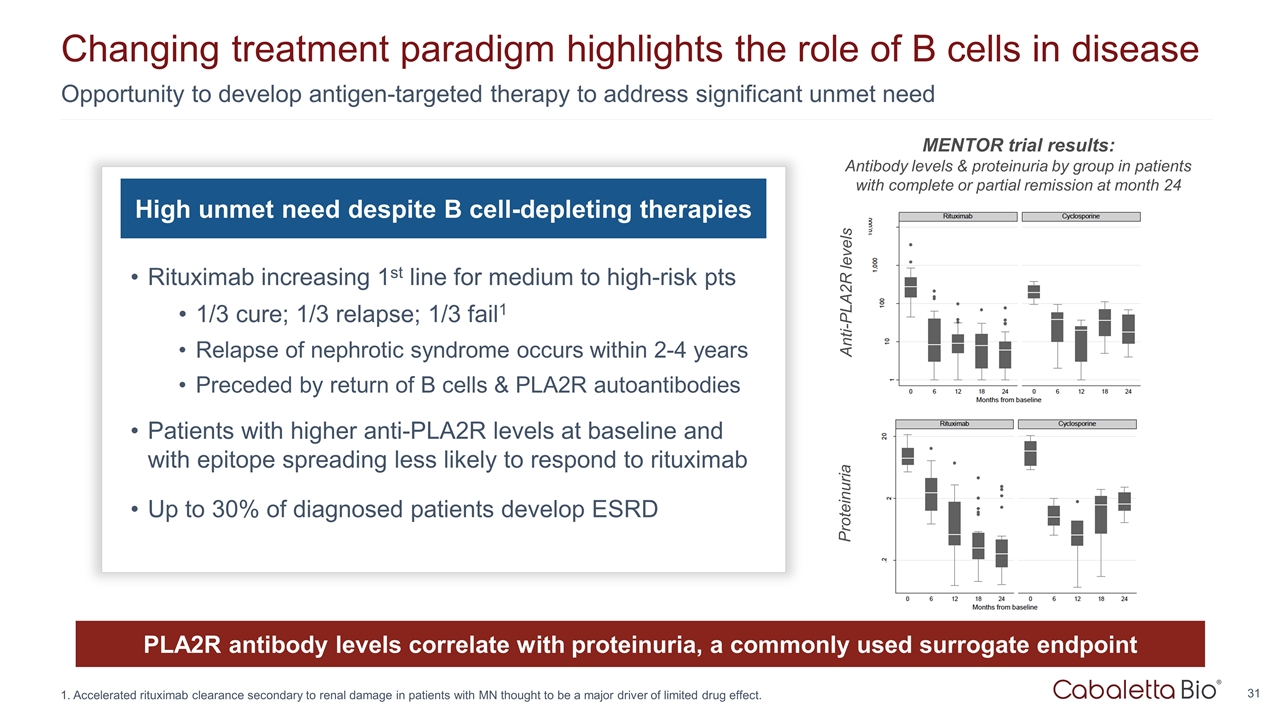
Opportunity to develop antigen-targeted therapy to address significant unmet need Changing treatment paradigm highlights the role of B cells in disease High unmet need despite B cell-depleting therapies Rituximab increasing 1st line for medium to high-risk pts 1/3 cure; 1/3 relapse; 1/3 fail1 Relapse of nephrotic syndrome occurs within 2-4 years Preceded by return of B cells & PLA2R autoantibodies Patients with higher anti-PLA2R levels at baseline and with epitope spreading less likely to respond to rituximab Up to 30% of diagnosed patients develop ESRD PLA2R antibody levels correlate with proteinuria, a commonly used surrogate endpoint Anti-PLA2R levels Proteinuria MENTOR trial results: Antibody levels & proteinuria by group in patients with complete or partial remission at month 24 Accelerated rituximab clearance secondary to renal damage in patients with MN thought to be a major driver of limited drug effect.
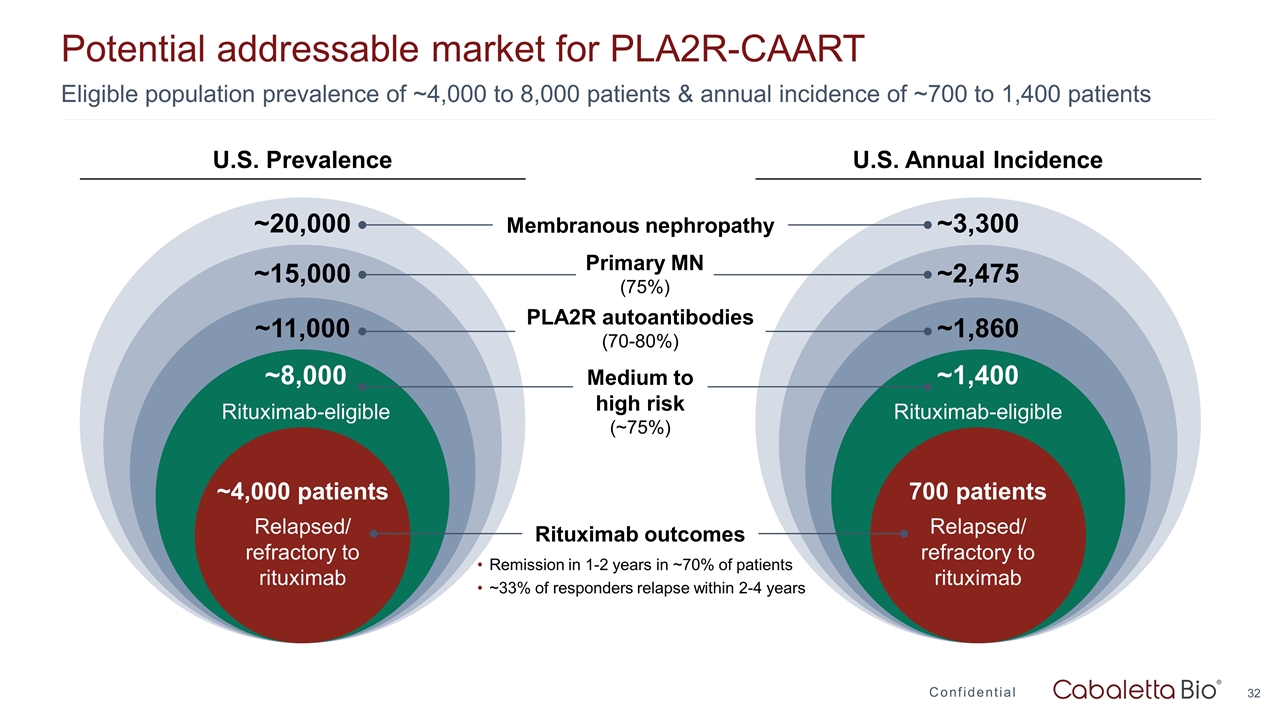
Potential addressable market for PLA2R-CAART Confidential Eligible population prevalence of ~4,000 to 8,000 patients & annual incidence of ~700 to 1,400 patients ~3,300 ~2,475 ~1,860 ~1,400 Rituximab-eligible 700 patients Relapsed/ refractory to rituximab ~20,000 ~15,000 ~11,000 ~8,000 Rituximab-eligible ~4,000 patients Relapsed/ refractory to rituximab Membranous nephropathy Primary MN (75%) PLA2R autoantibodies (70-80%) Medium to high risk (~75%) Rituximab outcomes Remission in 1-2 years in ~70% of patients ~33% of responders relapse within 2-4 years U.S. Prevalence U.S. Annual Incidence
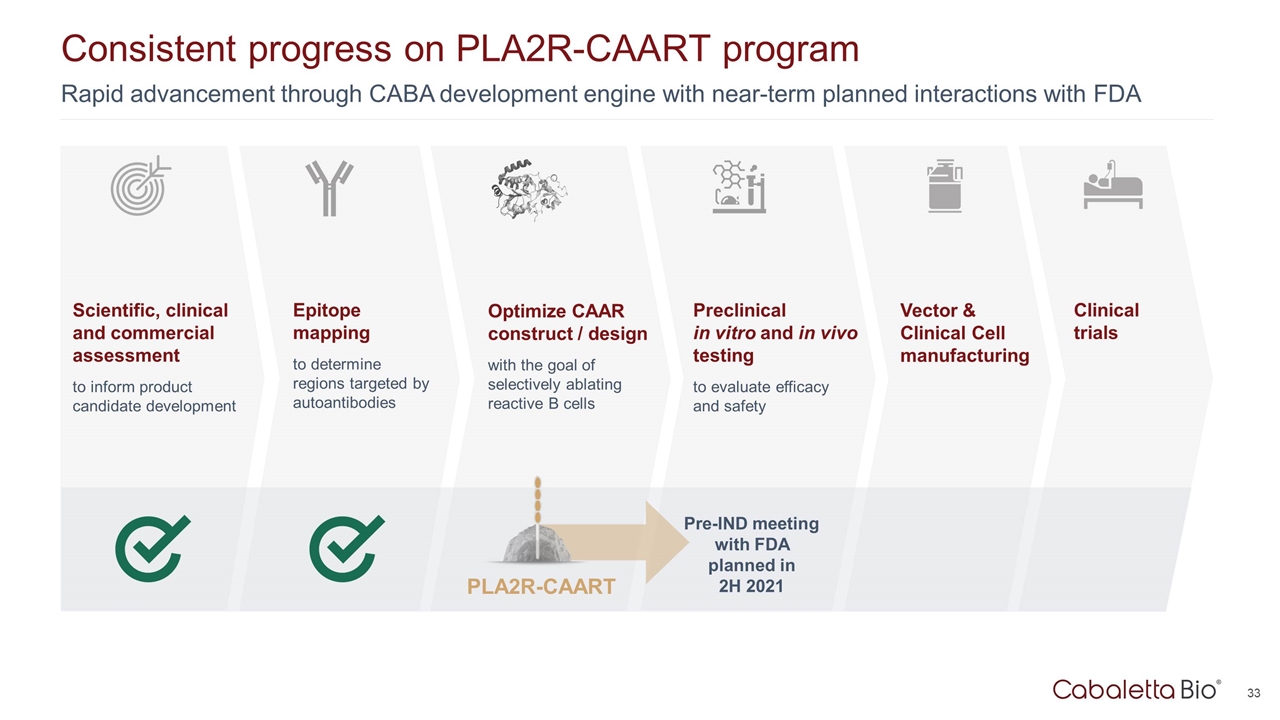
Consistent progress on PLA2R-CAART program Epitope mapping to determine regions targeted by autoantibodies Optimize CAAR construct / design with the goal of selectively ablating reactive B cells Preclinical in vitro and in vivo testing to evaluate efficacy and safety Scientific, clinical and commercial assessment to inform product candidate development Vector & Clinical Cell manufacturing Clinical trials Rapid advancement through CABA development engine with near-term planned interactions with FDA PLA2R-CAART Pre-IND meeting with FDA planned in 2H 2021

Manufacturing
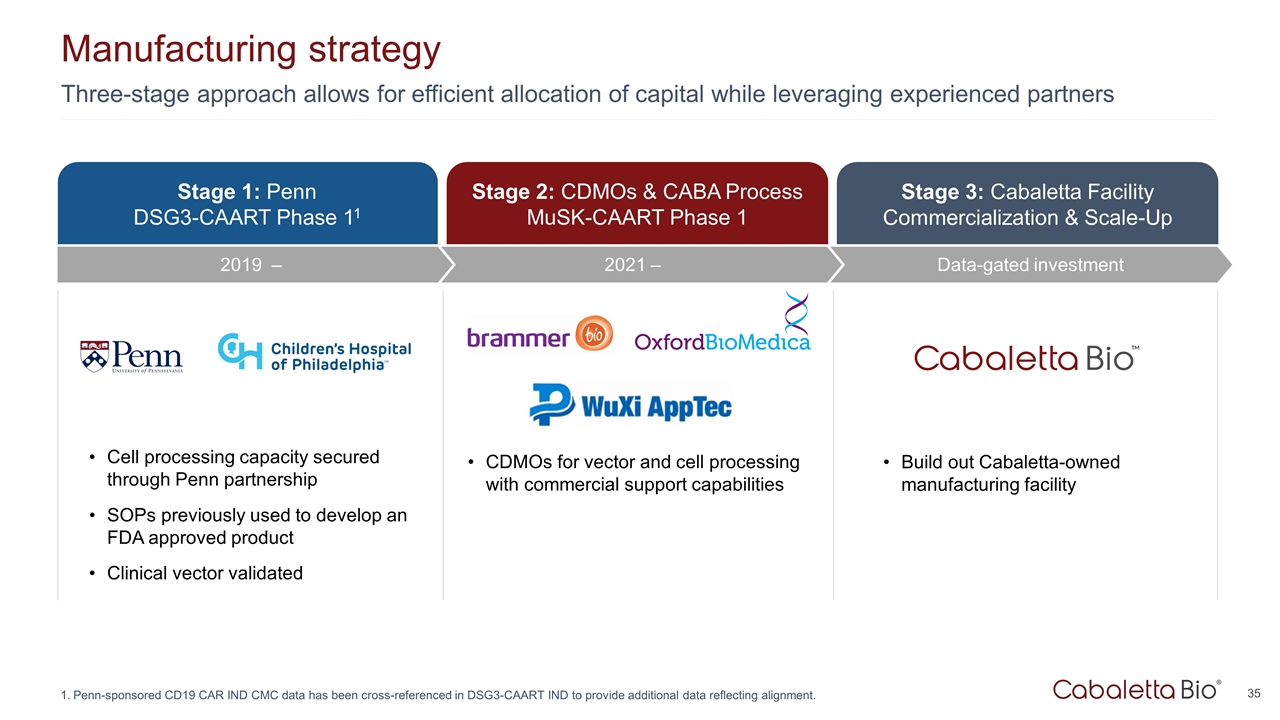
Three-stage approach allows for efficient allocation of capital while leveraging experienced partners Manufacturing strategy Penn-sponsored CD19 CAR IND CMC data has been cross-referenced in DSG3-CAART IND to provide additional data reflecting alignment. Stage 3: Cabaletta Facility Commercialization & Scale-Up Data-gated investment Stage 1: Penn DSG3-CAART Phase 11 Stage 2: CDMOs & CABA Process MuSK-CAART Phase 1 Cell processing capacity secured through Penn partnership SOPs previously used to develop an FDA approved product Clinical vector validated CDMOs for vector and cell processing with commercial support capabilities Build out Cabaletta-owned manufacturing facility 2021 – 2019 –
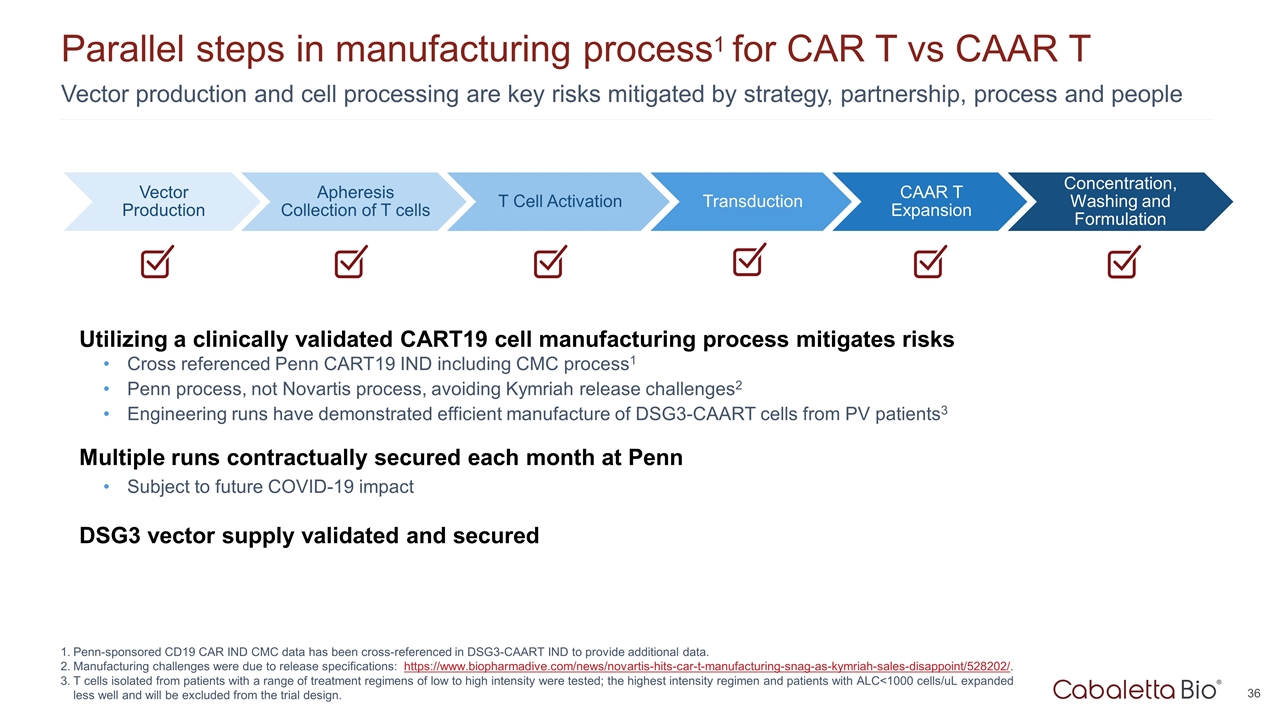
Utilizing a clinically validated CART19 cell manufacturing process mitigates risks Cross referenced Penn CART19 IND including CMC process1 Penn process, not Novartis process, avoiding Kymriah release challenges2 Engineering runs have demonstrated efficient manufacture of DSG3-CAART cells from PV patients3 Multiple runs contractually secured each month at Penn Subject to future COVID-19 impact DSG3 vector supply validated and secured Vector production and cell processing are key risks mitigated by strategy, partnership, process and people Parallel steps in manufacturing process1 for CAR T vs CAAR T Penn-sponsored CD19 CAR IND CMC data has been cross-referenced in DSG3-CAART IND to provide additional data. Manufacturing challenges were due to release specifications: https://www.biopharmadive.com/news/novartis-hits-car-t-manufacturing-snag-as-kymriah-sales-disappoint/528202/. T cells isolated from patients with a range of treatment regimens of low to high intensity were tested; the highest intensity regimen and patients with ALC<1000 cells/uL expanded less well and will be excluded from the trial design. Vector Production T Cell Activation Transduction CAAR T Expansion Concentration, Washing and Formulation Apheresis Collection of T cells

Corporate Summary

SCIENTIFIC ADVISORY BOARD Leadership team Aimee Payne, M.D., Ph.D. Co-Founder and Co-Chair Michael C. Milone, M.D., Ph.D. Co-Founder and Co-Chair Jay Siegel, M.D. LEADERSHIP TEAM Steven Nichtberger, M.D. President, CEO & Chairman Anup Marda Chief Financial Officer Arun Das, M.D. Executive Director BD Gwendolyn Binder, Ph.D. EVP Science & Technology David J. Chang, M.D., M.P.H. Chief Medical Officer J. Brian Stalter, J.D. General Counsel Martha O’Connor Chief HR Officer Iain McInnes, PhD, FRCP, FRSE, FMedSci Carl June, M.D.
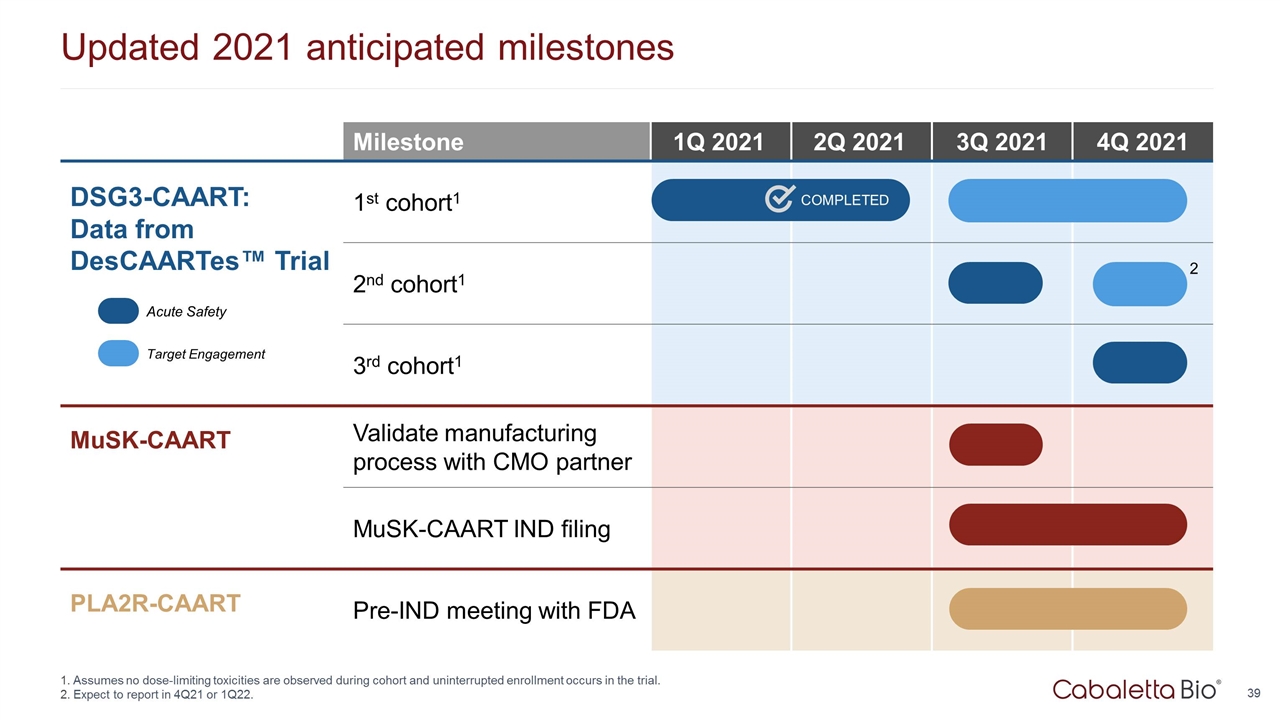
Updated 2021 anticipated milestones Milestone 1Q 2021 2Q 2021 3Q 2021 4Q 2021 DSG3-CAART: Data from DesCAARTes™ Trial 1st cohort1 2nd cohort1 3rd cohort1 MuSK-CAART Validate manufacturing process with CMO partner MuSK-CAART IND filing PLA2R-CAART Pre-IND meeting with FDA COMPLETED Acute Safety Target Engagement Assumes no dose-limiting toxicities are observed during cohort and uninterrupted enrollment occurs in the trial. Expect to report in 4Q21 or 1Q22. 2

Cabaletta Today Leveraging a clinically validated CAR T platform as a novel approach to treating autoimmune diseases aiming to provide: Deep and durable responses, potentially cures, for autoimmune patients Highly specific, targeted therapy designed to eliminate only pathogenic B cells Target engagement based on strength of biological rationale, deep understanding of translational data and many ways to deliver on the promise for patients Multiple potential near-term clinical data catalysts with potential for pipeline read-through Acute safety, target engagement, clinical responses Expanding network of academic & industry partners to enhance platform

Corporate Presentation May 2021