
Selective B cell ablation for autoimmune disease February 2020 Exhibit 99.1

The following presentation, including any printed or electronic copy of these slides, the talks given by the presenters, the information communicated during any delivery of the presentation and any question and answer session and any document or material distributed at or in connection with the presentation (collectively, the “Presentation”) has been prepared by Cabaletta Bio, Inc. (“we,” “us,” “our,” “Cabaletta” or the “Company”) and is made for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy securities, nor shall there be any sale of any securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. The Presentation does not purport to be a prospectus, to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this Presentation unless stated otherwise, and neither this Presentation, nor any sale of securities, shall under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This Presentation may contain “forward-looking statements” relating to our business, operations, and financial conditions, including, but not limited to, current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our preclinical and clinical results and other future conditions. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Factors which could cause actual results to differ materially from those in the forward-looking statements include, among others, the success, cost, and timing of our product candidate development activities and preclinical studies and clinical trials, our ability to obtain and maintain regulatory approval for our product candidates, our ability to commercialize our product candidates, future agreements with third parties in connection with the development or commercialization of our product candidates, the size and growth potential of the market for our product candidates, our ability to contract with third-party suppliers and manufacturers and our ability to develop internal manufacturing capabilities and facilities, the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing, and our ability to obtain and maintain intellectual property protection for our product candidates. Various risks, uncertainties and assumptions could cause actual results to differ materially from those anticipated or implied in our forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. The Company is the owner of various trademarks, trade names and service marks. Certain other trademarks, trade names and service marks appearing in this Presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this Presentation are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. Disclaimer

Develop and launch the first curative targeted cellular therapies for patients with autoimmune diseases Our vision
Developing CAAR T products (Chimeric AutoAntibody Receptor T) where there is a biologic opportunity for cure (over two dozen potential targets identified) by designing CAARs to selectively eliminate ONLY pathogenic B cells displaying targeted antibody while leveraging CART19 design and manufacturing experience from Penn DesCAARTes Phase 1 trial – DSG3-CAART in mucosal pemphigus vulgaris (PV) patients progressing to support expected timelines Orphan Drug Designation granted by the FDA in January 2020 for the treatment of PV CABA Labs leading evaluation and development of risk mitigating approaches Expanding product pipeline with our CABA platform (Cabaletta Approach for selective B cell Ablation) preclinical data published on PV, myasthenia gravis, and hemophilia A inhibitor robust IP portfolio emerging with first US patent issued on lead program Planned catalysts in 2020 DesCAARTes trial – Report clinical acute tolerability (8 day) data from initial cohort MuSK-CAART – Confirm in vivo target engagement and initiate IND-enabling studies Manufacturing independence – Initiate validation of manufacturing for clinical trials with CMO1 Key messages Leveraging CAR T experience to specifically target B cell-mediated autoimmune diseases Contract Manufacturing Organization

Diversified with long-standing history of professional collaborations among team and with co-founders Leadership Steven Nichtberger, MD President, CEO & Chairman Scientific Advisory Board Board of Directors Michael Milone, MD PhD Co-chair, Co-founder Aimee Payne, MD PhD Co-chair, Co-founder Jay Siegel, MD Catherine Bollard, MD PhD Mark Simon Brian Daniels, MD Richard Henriques Gwendolyn Binder, PhD EVP Science & Technology David Chang, MD Chief Medical Officer Anup Marda Chief Financial Officer Brian Stalter General Counsel Arun Das, MD Senior Director BD Martha O’Connor Chief HR Officer
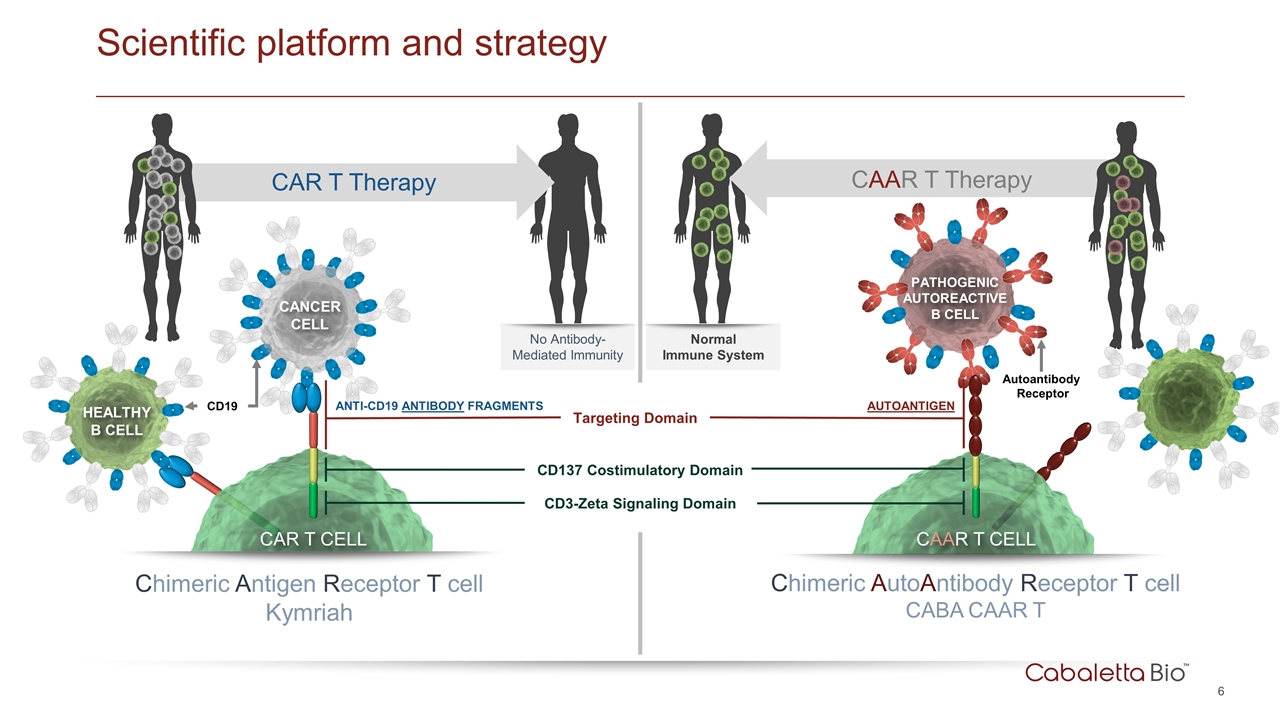
Scientific platform and strategy CAR T CELL CD19 CANCER CELL Chimeric Antigen Receptor T cell Kymriah Targeting Domain ANTI-CD19 ANTIBODY FRAGMENTS HEALTHY B CELL CD137 Costimulatory Domain CD3-Zeta Signaling Domain CAR T Therapy No Antibody-Mediated Immunity CAAR T Therapy Normal Immune System CAAR T CELL Chimeric AutoAntibody Receptor T cell CABA CAAR T PATHOGENIC AUTOREACTIVE B CELL AUTOANTIGEN Autoantibody Receptor HEALTHY B CELL
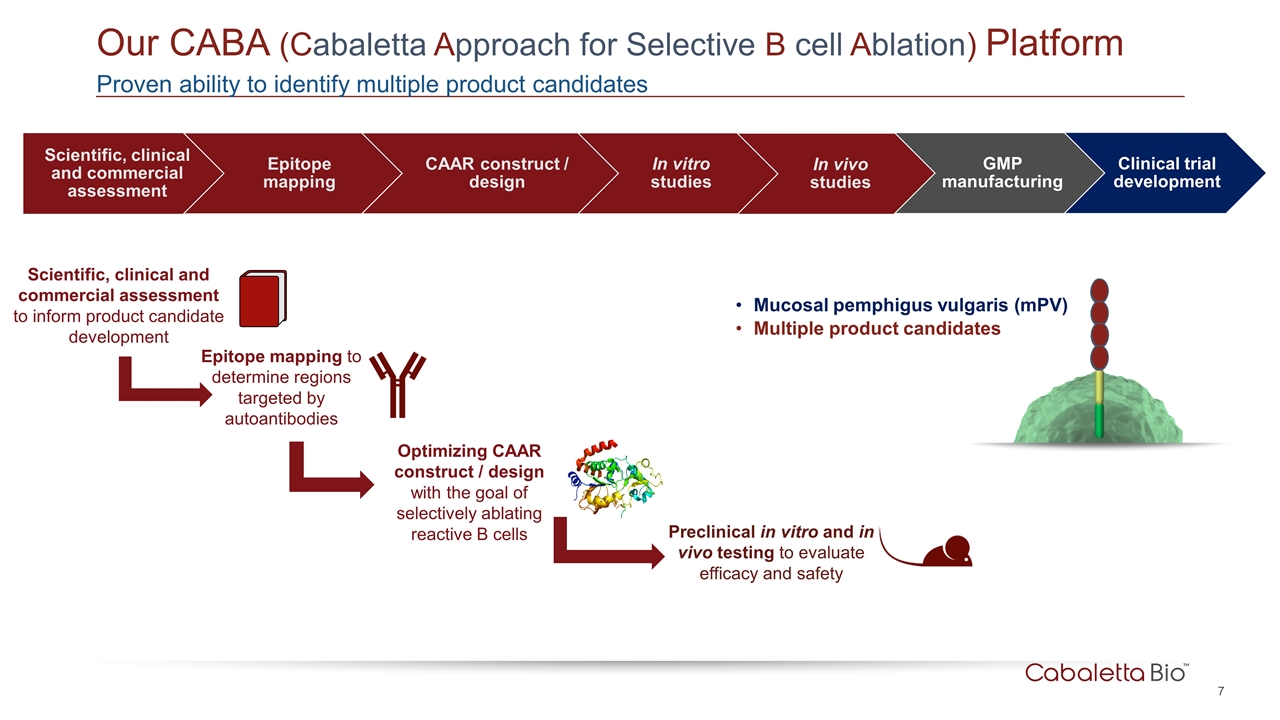
Our CABA (Cabaletta Approach for Selective B cell Ablation) Platform Proven ability to identify multiple product candidates Epitope mapping to determine regions targeted by autoantibodies Optimizing CAAR construct / design with the goal of selectively ablating reactive B cells Preclinical in vitro and in vivo testing to evaluate efficacy and safety Scientific, clinical and commercial assessment to inform product candidate development Mucosal pemphigus vulgaris (mPV) Multiple product candidates Epitope mapping CAAR construct / design In vitro studies In vivo studies GMP manufacturing Clinical trial development Scientific, clinical and commercial assessment
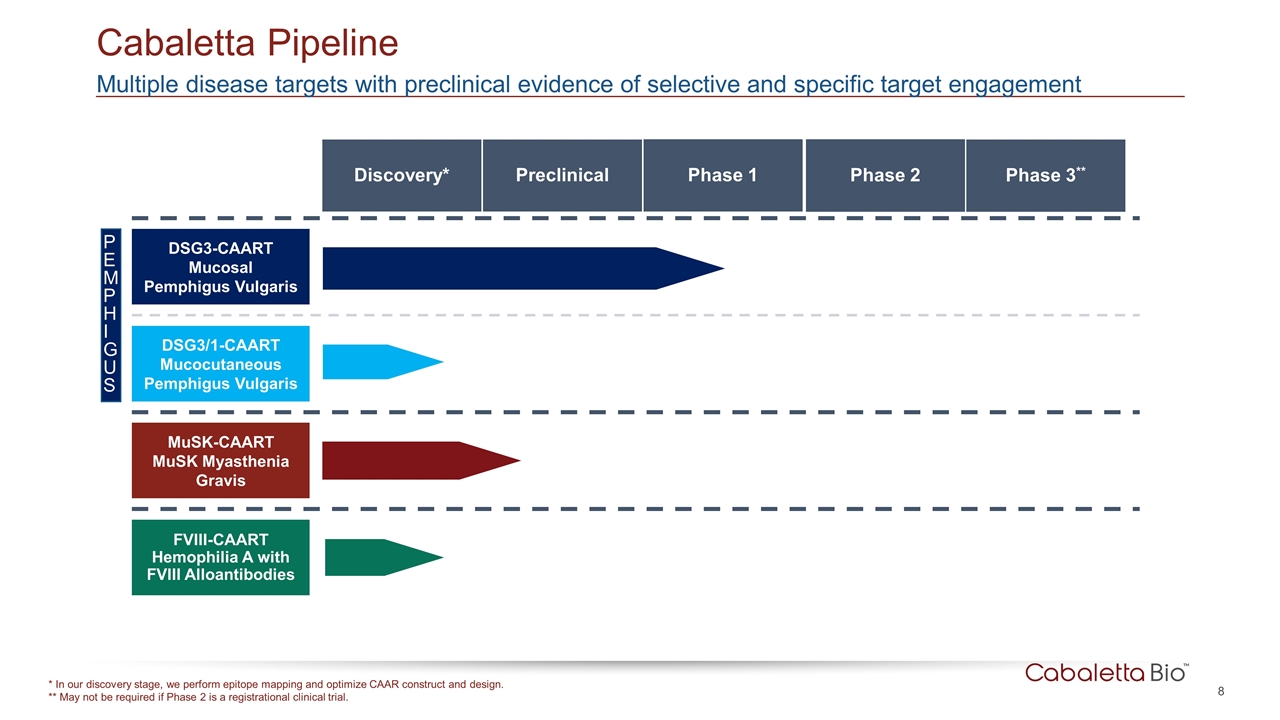
Cabaletta Pipeline * In our discovery stage, we perform epitope mapping and optimize CAAR construct and design. ** May not be required if Phase 2 is a registrational clinical trial. Multiple disease targets with preclinical evidence of selective and specific target engagement DSG3-CAART Mucosal Pemphigus Vulgaris DSG3/1-CAART Mucocutaneous Pemphigus Vulgaris MuSK-CAART MuSK Myasthenia Gravis FVIII-CAART Hemophilia A with FVIII Alloantibodies PEMPHIGUS Phase 1 Phase 2 Phase 3** Preclinical Discovery*
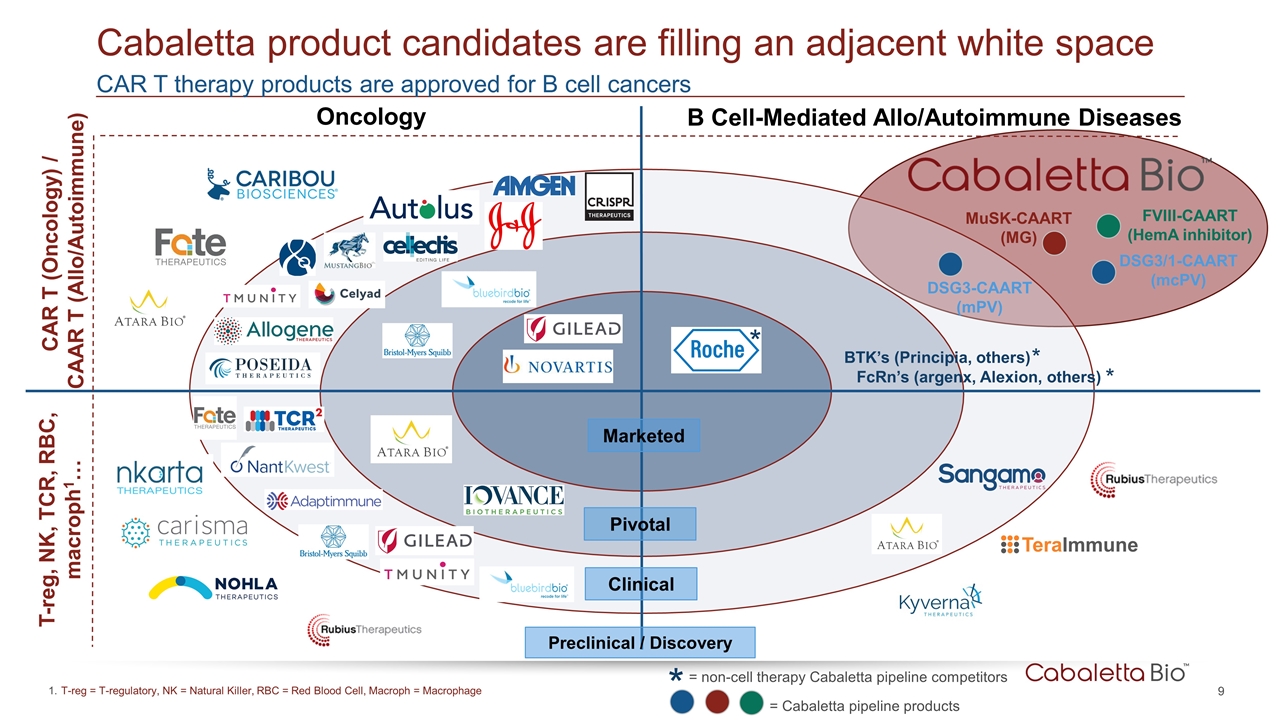
CAR T therapy products are approved for B cell cancers Cabaletta product candidates are filling an adjacent white space Clinical Pivotal Marketed Oncology B Cell-Mediated Allo/Autoimmune Diseases DSG3-CAART (mPV) MuSK-CAART (MG) FVIII-CAART (HemA inhibitor) DSG3/1-CAART (mcPV) Preclinical / Discovery FcRn’s (argenx, Alexion, others) BTK’s (Principia, others) * * * T-reg = T-regulatory, NK = Natural Killer, RBC = Red Blood Cell, Macroph = Macrophage = Cabaletta pipeline products * = non-cell therapy Cabaletta pipeline competitors CAR T (Oncology) / CAAR T (Allo/Autoimmune) T-reg, NK, TCR, RBC, macroph1…
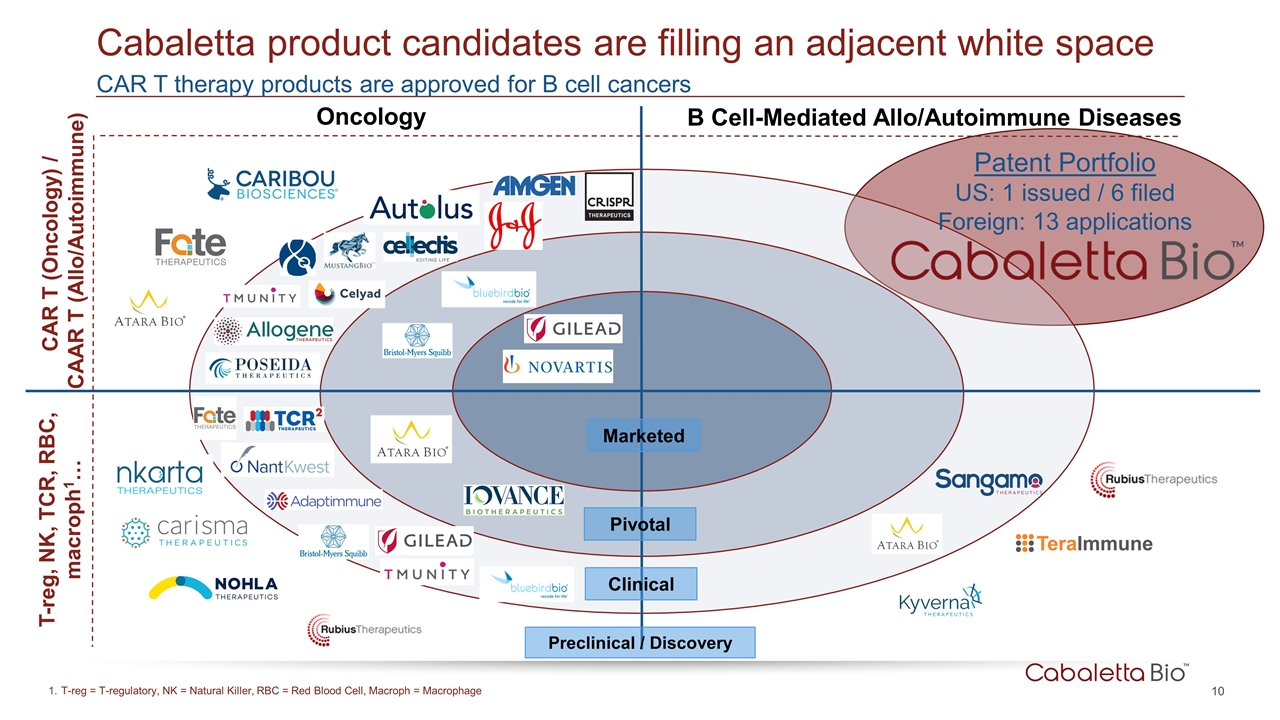
CAR T therapy products are approved for B cell cancers Cabaletta product candidates are filling an adjacent white space Clinical Pivotal Marketed CAR T (Oncology) / CAAR T (Allo/Autoimmune) T-reg, NK, TCR, RBC, macroph1… Oncology B Cell-Mediated Allo/Autoimmune Diseases Preclinical / Discovery Patent Portfolio US: 1 issued / 6 filed Foreign: 13 applications T-reg = T-regulatory, NK = Natural Killer, RBC = Red Blood Cell, Macroph = Macrophage

Patent rights pursued on a target by target basis Pursuing multiple levels of protection for CAAR constructs protein, nucleic acid, and engineered cells; and their use Expiration Dates: 2035-2039 (without patent term extensions) First issued US patent for CAAR therapy in pemphigus Exemplary Composition of Matter Claim “A genetically modified cell comprising a CAAR comprising an extracellular domain comprising DSG1, DSG3, or a fragment thereof that binds an autoantibody expressed on a B-cell, a transmembrane domain, and an intracellular signaling domain, wherein the cell expresses the CAAR and binds the autoantibody expressed on the B cell or induces killing of the B cell expressing the autoantibody.” Patent rights owned by the University of Pennsylvania (Penn) or co-owned by Penn and the Children’s Hospital of Philadelphia and exclusively licensed globally to Cabaletta Distinct from CD19 directed CAR T patents, initial CAAR T patent covers entire human antigen Overview of Intellectual Property Strategy

DSG3-CAART for mucosal pemphigus vulgaris patients

Pemphigus Vulgaris CAAR T Preclinical Development (video) Ellebrecht…Milone, Payne, Science 2016 Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease Christoph T. Ellebrecht,1 Vijay G. Bhoj,2 Arben Nace,1 Eun Jung Choi,1 Xuming Mao,1 Michael Jeffrey Cho,1 Giovanni Di Zenzo,3 Antonio Lanzavecchia,4 John T. Seykora,1 George Cotsarelis,1 Michael C. Milone,2*† Aimee S. Payne1*†
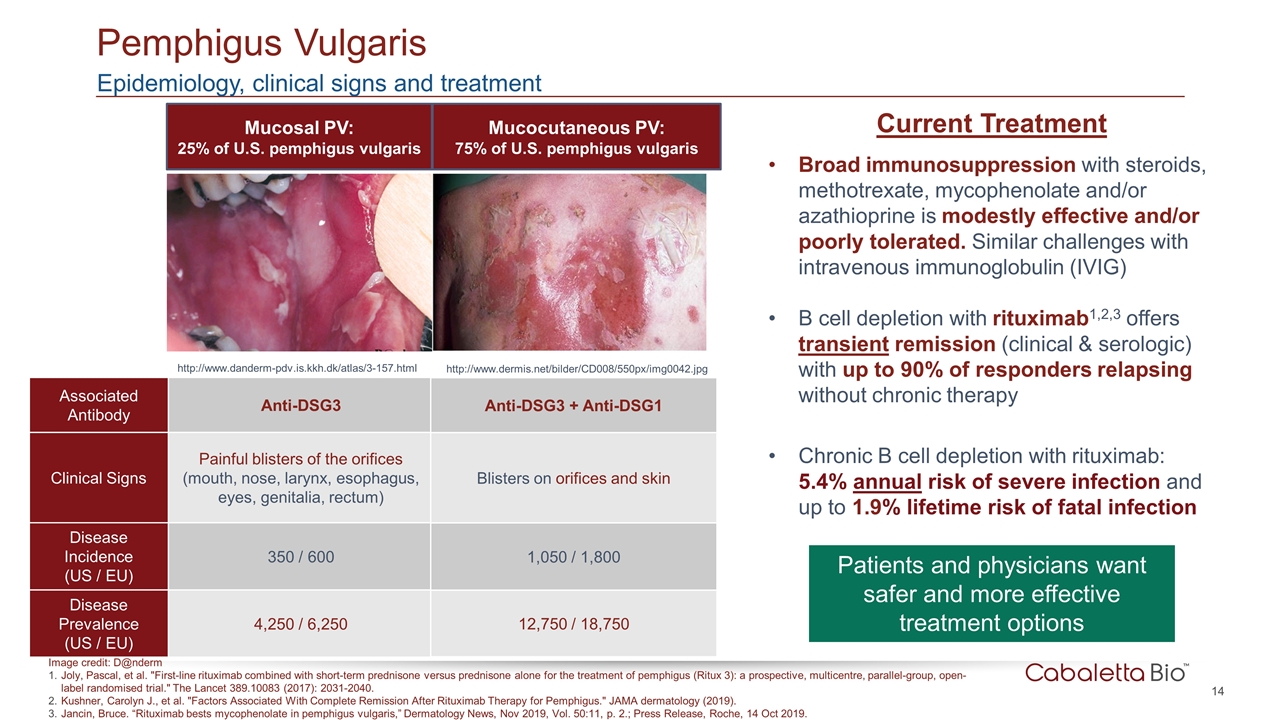
Pemphigus Vulgaris Mucosal PV: 25% of U.S. pemphigus vulgaris Mucocutaneous PV: 75% of U.S. pemphigus vulgaris http://www.danderm-pdv.is.kkh.dk/atlas/3-157.html http://www.dermis.net/bilder/CD008/550px/img0042.jpg Associated Antibody Anti-DSG3 Clinical Signs Painful blisters of the orifices (mouth, nose, larynx, esophagus, eyes, genitalia, rectum) Disease Incidence (US / EU) 350 / 600 Disease Prevalence (US / EU) 4,250 / 6,250 Current Treatment Broad immunosuppression with steroids, methotrexate, mycophenolate and/or azathioprine is modestly effective and/or poorly tolerated. Similar challenges with intravenous immunoglobulin (IVIG) B cell depletion with rituximab1,2,3 offers transient remission (clinical & serologic) with up to 90% of responders relapsing without chronic therapy Chronic B cell depletion with rituximab: 5.4% annual risk of severe infection and up to 1.9% lifetime risk of fatal infection Patients and physicians want safer and more effective treatment options Anti-DSG3 + Anti-DSG1 Blisters on orifices and skin 1,050 / 1,800 12,750 / 18,750 Epidemiology, clinical signs and treatment Image credit: D@nderm Joly, Pascal, et al. "First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial." The Lancet 389.10083 (2017): 2031-2040. Kushner, Carolyn J., et al. "Factors Associated With Complete Remission After Rituximab Therapy for Pemphigus." JAMA dermatology (2019). Jancin, Bruce. “Rituximab bests mycophenolate in pemphigus vulgaris,” Dermatology News, Nov 2019, Vol. 50:11, p. 2.; Press Release, Roche, 14 Oct 2019.
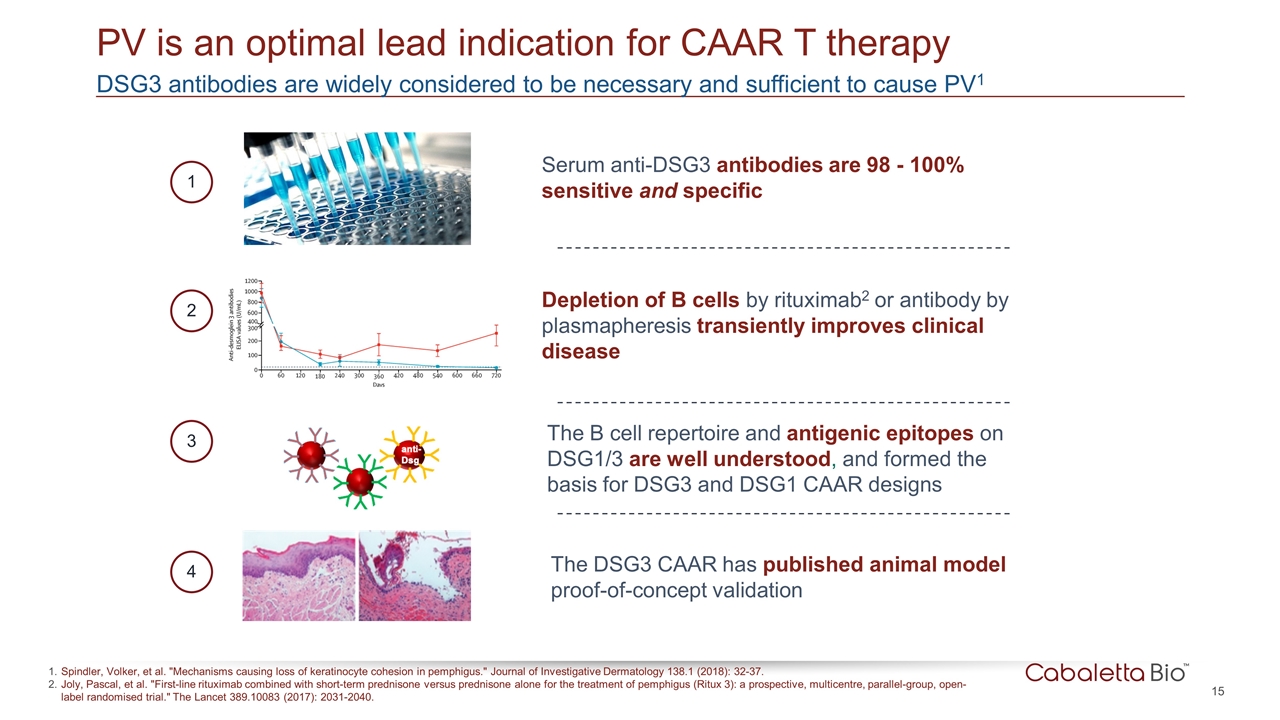
DSG3 antibodies are widely considered to be necessary and sufficient to cause PV1 PV is an optimal lead indication for CAAR T therapy Serum anti-DSG3 antibodies are 98 - 100% sensitive and specific The DSG3 CAAR has published animal model proof-of-concept validation Depletion of B cells by rituximab2 or antibody by plasmapheresis transiently improves clinical disease The B cell repertoire and antigenic epitopes on DSG1/3 are well understood, and formed the basis for DSG3 and DSG1 CAAR designs 1 2 3 4 Spindler, Volker, et al. "Mechanisms causing loss of keratinocyte cohesion in pemphigus." Journal of Investigative Dermatology 138.1 (2018): 32-37. Joly, Pascal, et al. "First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial." The Lancet 389.10083 (2017): 2031-2040.
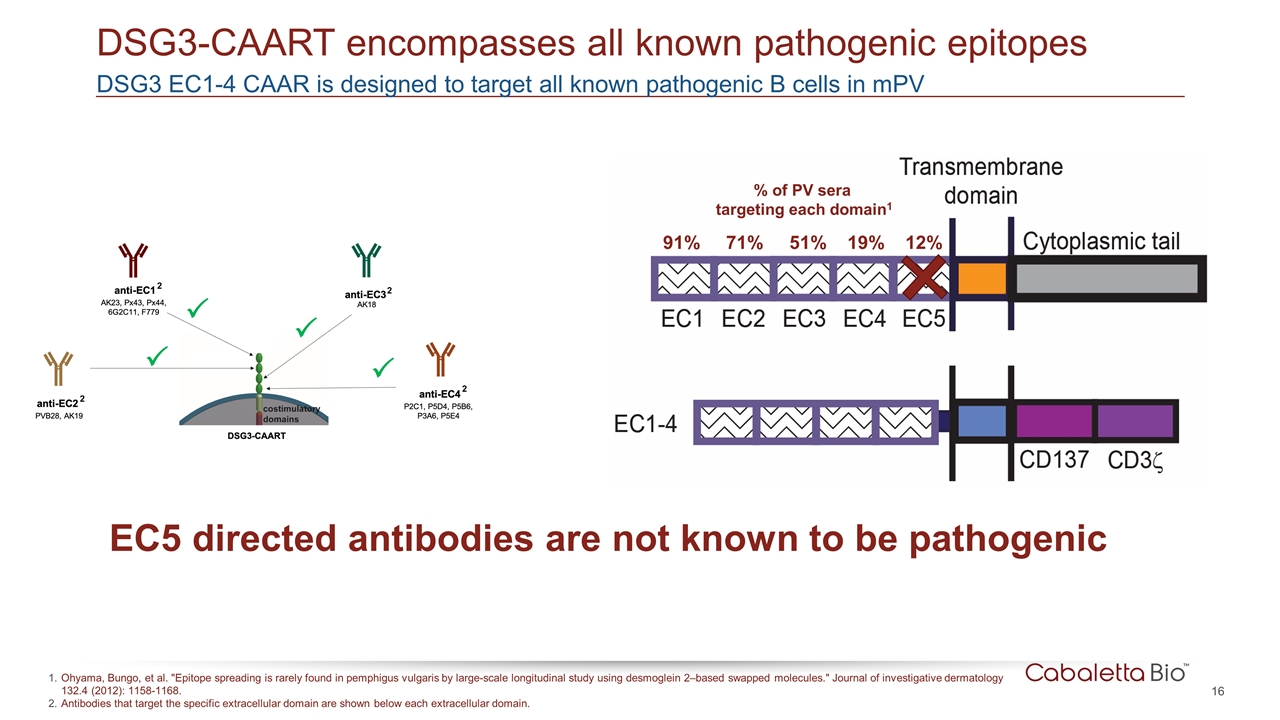
DSG3 EC1-4 CAAR is designed to target all known pathogenic B cells in mPV DSG3-CAART encompasses all known pathogenic epitopes Ohyama, Bungo, et al. "Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudinal study using desmoglein 2–based swapped molecules." Journal of investigative dermatology 132.4 (2012): 1158-1168. Antibodies that target the specific extracellular domain are shown below each extracellular domain. 91% 71% 51% 19% 12% % of PV sera targeting each domain1 EC5 directed antibodies are not known to be pathogenic 2 2 2 2
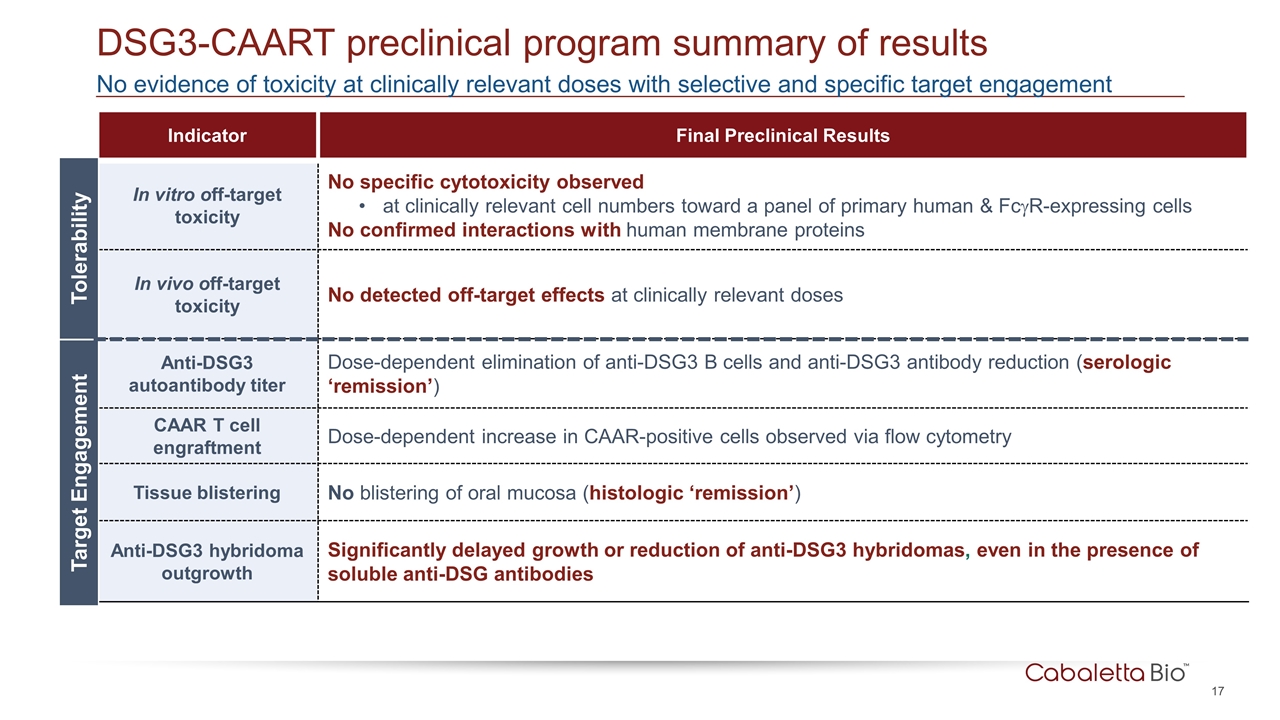
No evidence of toxicity at clinically relevant doses with selective and specific target engagement DSG3-CAART preclinical program summary of results Indicator Final Preclinical Results In vitro off-target toxicity No specific cytotoxicity observed at clinically relevant cell numbers toward a panel of primary human & FcgR-expressing cells No confirmed interactions with human membrane proteins In vivo off-target toxicity No detected off-target effects at clinically relevant doses Anti-DSG3 autoantibody titer Dose-dependent elimination of anti-DSG3 B cells and anti-DSG3 antibody reduction (serologic ‘remission’) CAAR T cell engraftment Dose-dependent increase in CAAR-positive cells observed via flow cytometry Tissue blistering No blistering of oral mucosa (histologic ‘remission’) Anti-DSG3 hybridoma outgrowth Significantly delayed growth or reduction of anti-DSG3 hybridomas, even in the presence of soluble anti-DSG antibodies Tolerability Target Engagement
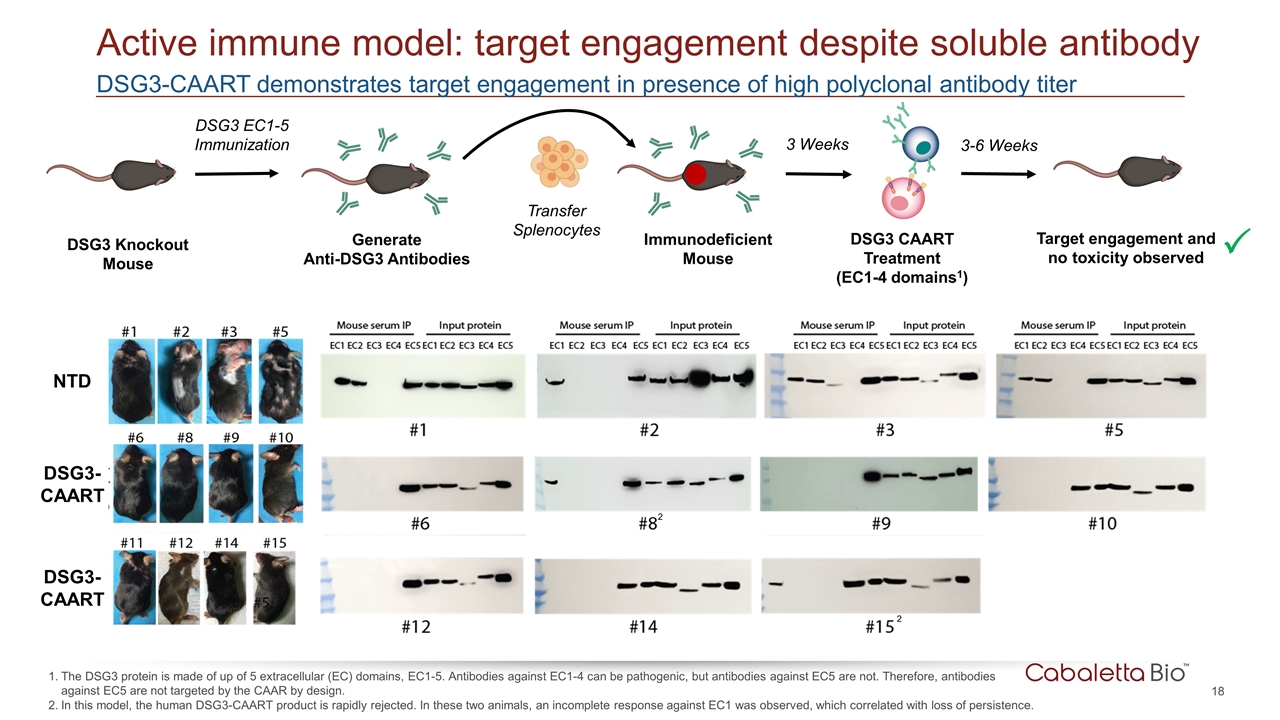
DSG3-CAART demonstrates target engagement in presence of high polyclonal antibody titer Active immune model: target engagement despite soluble antibody The DSG3 protein is made of up of 5 extracellular (EC) domains, EC1-5. Antibodies against EC1-4 can be pathogenic, but antibodies against EC5 are not. Therefore, antibodies against EC5 are not targeted by the CAAR by design. In this model, the human DSG3-CAART product is rapidly rejected. In these two animals, an incomplete response against EC1 was observed, which correlated with loss of persistence. DSG3 Knockout Mouse DSG3 EC1-5 Immunization Generate Anti-DSG3 Antibodies Transfer Splenocytes Immunodeficient Mouse NTD DSG3- CAART DSG3- CAART 3 Weeks Target engagement and no toxicity observed 3-6 Weeks P DSG3 CAART Treatment (EC1-4 domains1) 2 2
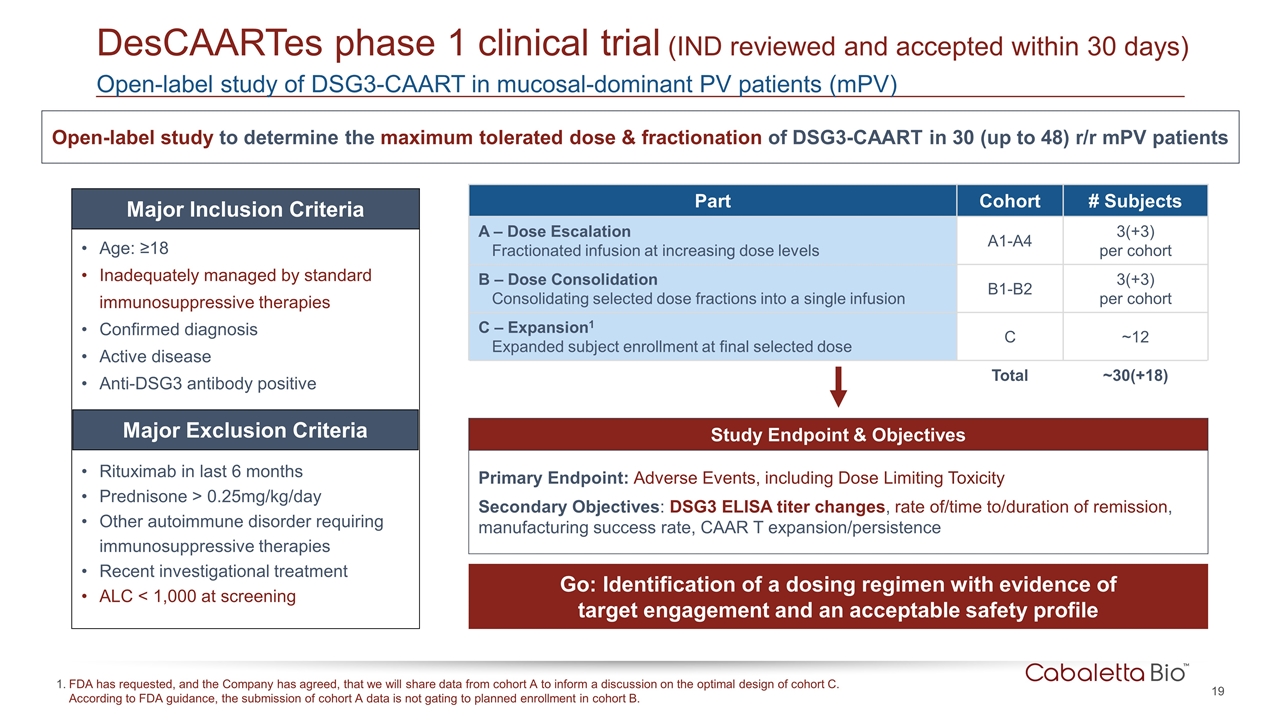
Study Endpoint & Objectives Primary Endpoint: Adverse Events, including Dose Limiting Toxicity Secondary Objectives: DSG3 ELISA titer changes, rate of/time to/duration of remission, manufacturing success rate, CAAR T expansion/persistence Open-label study of DSG3-CAART in mucosal-dominant PV patients (mPV) DesCAARTes phase 1 clinical trial (IND reviewed and accepted within 30 days) Open-label study to determine the maximum tolerated dose & fractionation of DSG3-CAART in 30 (up to 48) r/r mPV patients Go: Identification of a dosing regimen with evidence of target engagement and an acceptable safety profile Part Cohort # Subjects A – Dose Escalation Fractionated infusion at increasing dose levels A1-A4 3(+3) per cohort B – Dose Consolidation Consolidating selected dose fractions into a single infusion B1-B2 3(+3) per cohort C – Expansion1 Expanded subject enrollment at final selected dose C ~12 Total ~30(+18) Age: ≥18 Inadequately managed by standard immunosuppressive therapies Confirmed diagnosis Active disease Anti-DSG3 antibody positive Rituximab in last 6 months Prednisone > 0.25mg/kg/day Other autoimmune disorder requiring immunosuppressive therapies Recent investigational treatment ALC < 1,000 at screening Major Inclusion Criteria Major Exclusion Criteria FDA has requested, and the Company has agreed, that we will share data from cohort A to inform a discussion on the optimal design of cohort C. According to FDA guidance, the submission of cohort A data is not gating to planned enrollment in cohort B.
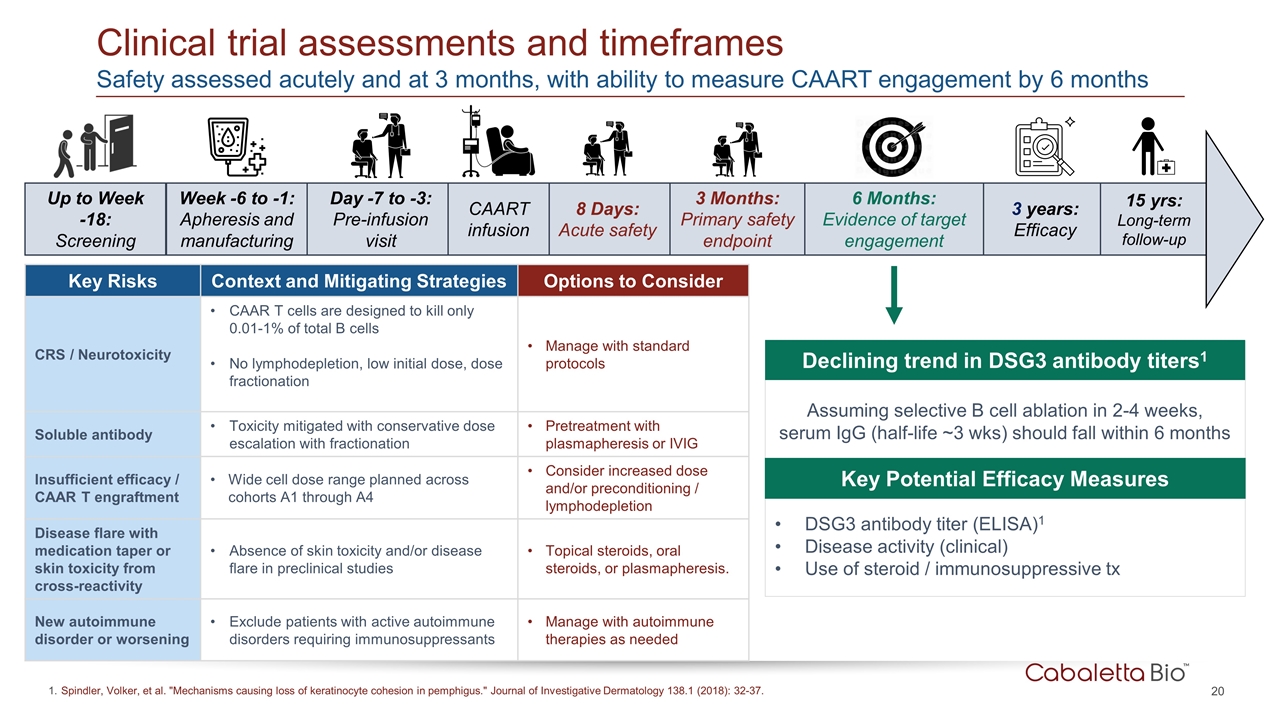
8 Days: Acute safety Up to Week -18: Screening Day -7 to -3: Pre-infusion visit 3 Months: Primary safety endpoint 3 years: Efficacy 15 yrs: Long-term follow-up CAART infusion Week -6 to -1: Apheresis and manufacturing Clinical trial assessments and timeframes Spindler, Volker, et al. "Mechanisms causing loss of keratinocyte cohesion in pemphigus." Journal of Investigative Dermatology 138.1 (2018): 32-37. 6 Months: Evidence of target engagement Safety assessed acutely and at 3 months, with ability to measure CAART engagement by 6 months Key Risks Context and Mitigating Strategies Options to Consider CRS / Neurotoxicity CAAR T cells are designed to kill only 0.01-1% of total B cells No lymphodepletion, low initial dose, dose fractionation Manage with standard protocols Soluble antibody Toxicity mitigated with conservative dose escalation with fractionation Pretreatment with plasmapheresis or IVIG Insufficient efficacy / CAAR T engraftment Wide cell dose range planned across cohorts A1 through A4 Consider increased dose and/or preconditioning / lymphodepletion Disease flare with medication taper or skin toxicity from cross-reactivity Absence of skin toxicity and/or disease flare in preclinical studies Topical steroids, oral steroids, or plasmapheresis. New autoimmune disorder or worsening Exclude patients with active autoimmune disorders requiring immunosuppressants Manage with autoimmune therapies as needed Assuming selective B cell ablation in 2-4 weeks, serum IgG (half-life ~3 wks) should fall within 6 months DSG3 antibody titer (ELISA)1 Disease activity (clinical) Use of steroid / immunosuppressive tx Key Potential Efficacy Measures Declining trend in DSG3 antibody titers1

Enrollment Patients dosed over 1-3 weeks for initial cohorts, then followed for at least 2-4 weeks before next patient Timelines may be delayed due to non-material changes or challenges, including among others: identifying appropriate patients for 1st trial of cell therapy in autoimmune disease mitigating risks observed in earlier patients manufacturing prioritization or outcomes Safety updates by dosing cohort – limited top-line updates Acute – within 8 days of infusion Primary endpoint safety measures – 3 months Target engagement updates by dosing cohort – possible but not expected in lowest dose DSG3 antibody is necessary and sufficient to cause mPV (per consensus document1) DSG3 antibody half-life ~3 weeks Potential evidence of reduction of DSG3 antibody levels within six months Clinical responses including mucosal lesion response and absence of recurrence may take longer By dosing cohort except for serious adverse events that materially change timelines or protocol Clinical trial communication plan Spindler V, Eming R, Schmidt E, et al. Mechanisms Causing Loss of Keratinocyte Cohesion in Pemphigus. J Invest Dermatol 2018;138:32-7.
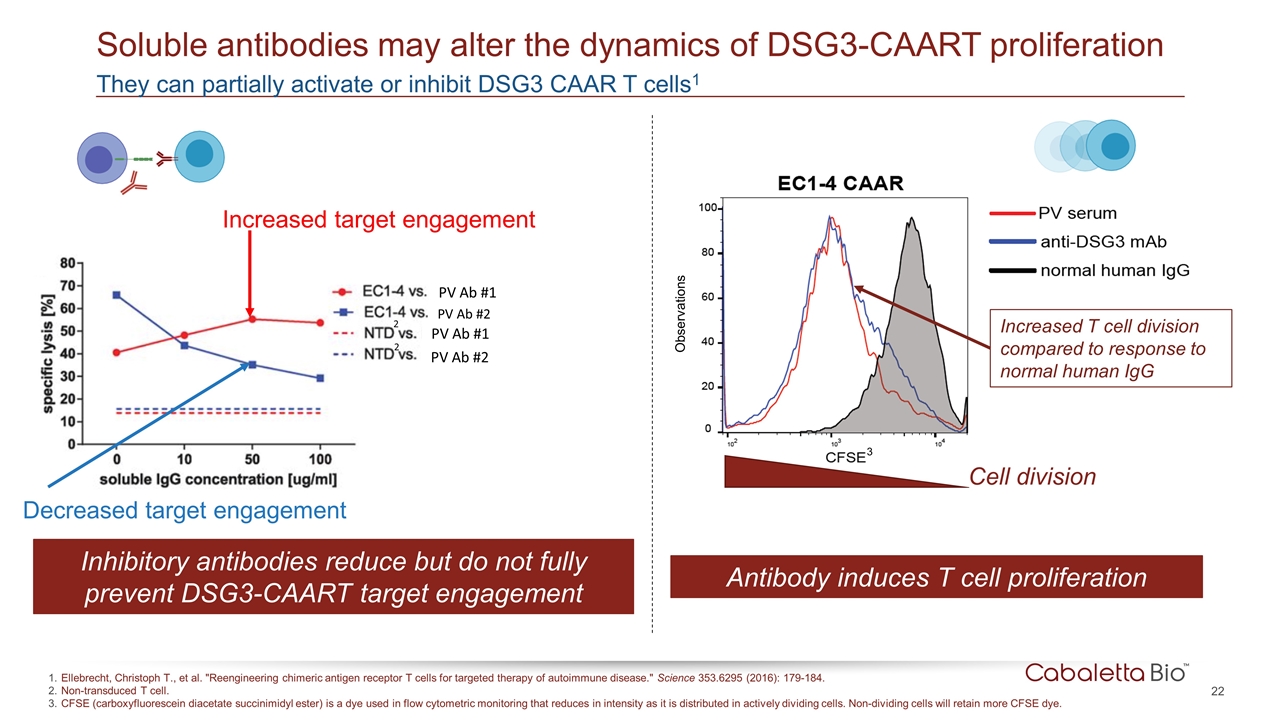
They can partially activate or inhibit DSG3 CAAR T cells1 Soluble antibodies may alter the dynamics of DSG3-CAART proliferation Ellebrecht, Christoph T., et al. "Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease." Science 353.6295 (2016): 179-184. Non-transduced T cell. CFSE (carboxyfluorescein diacetate succinimidyl ester) is a dye used in flow cytometric monitoring that reduces in intensity as it is distributed in actively dividing cells. Non-dividing cells will retain more CFSE dye. Increased target engagement Decreased target engagement PV Ab #1 PV Ab #2 PV Ab #1 PV Ab #2 Cell division Antibody induces T cell proliferation Inhibitory antibodies reduce but do not fully prevent DSG3-CAART target engagement 2 2 Increased T cell division compared to response to normal human IgG 3 Observations
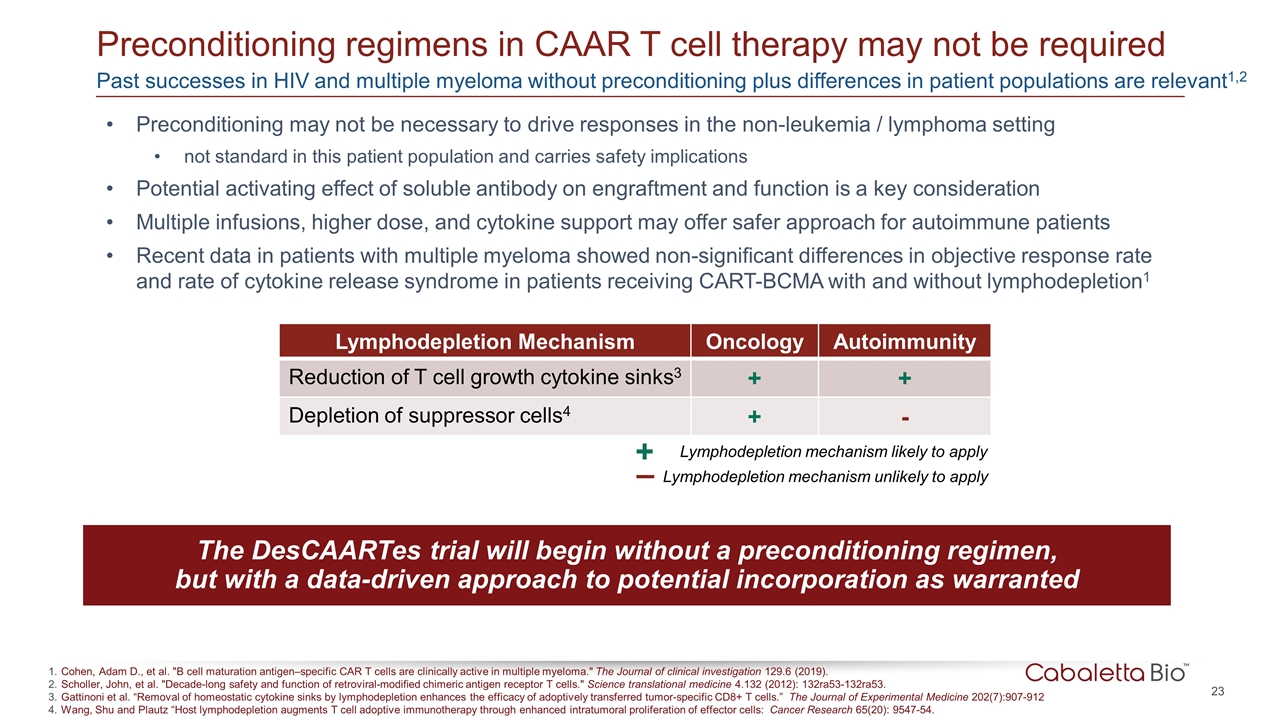
The DesCAARTes trial will begin without a preconditioning regimen, but with a data-driven approach to potential incorporation as warranted Lymphodepletion Mechanism Oncology Autoimmunity Reduction of T cell growth cytokine sinks3 + + Depletion of suppressor cells4 + - Preconditioning regimens in CAAR T cell therapy may not be required Cohen, Adam D., et al. "B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma." The Journal of clinical investigation 129.6 (2019). Scholler, John, et al. "Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells." Science translational medicine 4.132 (2012): 132ra53-132ra53. Gattinoni et al. “Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells.” The Journal of Experimental Medicine 202(7):907-912 Wang, Shu and Plautz “Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells: Cancer Research 65(20): 9547-54. Preconditioning may not be necessary to drive responses in the non-leukemia / lymphoma setting not standard in this patient population and carries safety implications Potential activating effect of soluble antibody on engraftment and function is a key consideration Multiple infusions, higher dose, and cytokine support may offer safer approach for autoimmune patients Recent data in patients with multiple myeloma showed non-significant differences in objective response rate and rate of cytokine release syndrome in patients receiving CART-BCMA with and without lymphodepletion1 – Lymphodepletion mechanism unlikely to apply + Lymphodepletion mechanism likely to apply Past successes in HIV and multiple myeloma without preconditioning plus differences in patient populations are relevant1,2

MuSK-CAART for myasthenia gravis patients
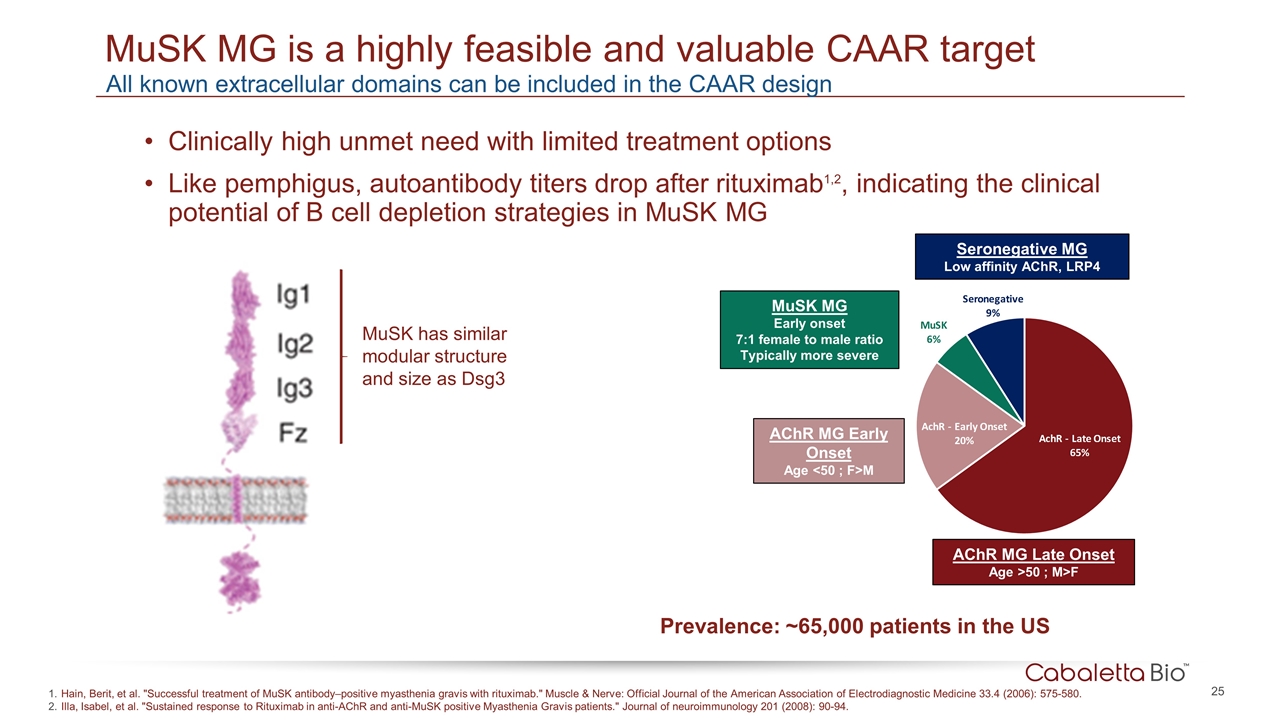
MuSK MG is a highly feasible and valuable CAAR target Clinically high unmet need with limited treatment options Like pemphigus, autoantibody titers drop after rituximab1,2, indicating the clinical potential of B cell depletion strategies in MuSK MG MuSK has similar modular structure and size as Dsg3 Hain, Berit, et al. "Successful treatment of MuSK antibody–positive myasthenia gravis with rituximab." Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 33.4 (2006): 575-580. Illa, Isabel, et al. "Sustained response to Rituximab in anti-AChR and anti-MuSK positive Myasthenia Gravis patients." Journal of neuroimmunology 201 (2008): 90-94. All known extracellular domains can be included in the CAAR design AChR MG Early Onset Age <50 ; F>M AChR MG Late Onset Age >50 ; M>F MuSK MG Early onset 7:1 female to male ratio Typically more severe Seronegative MG Low affinity AChR, LRP4 Prevalence: ~65,000 patients in the US
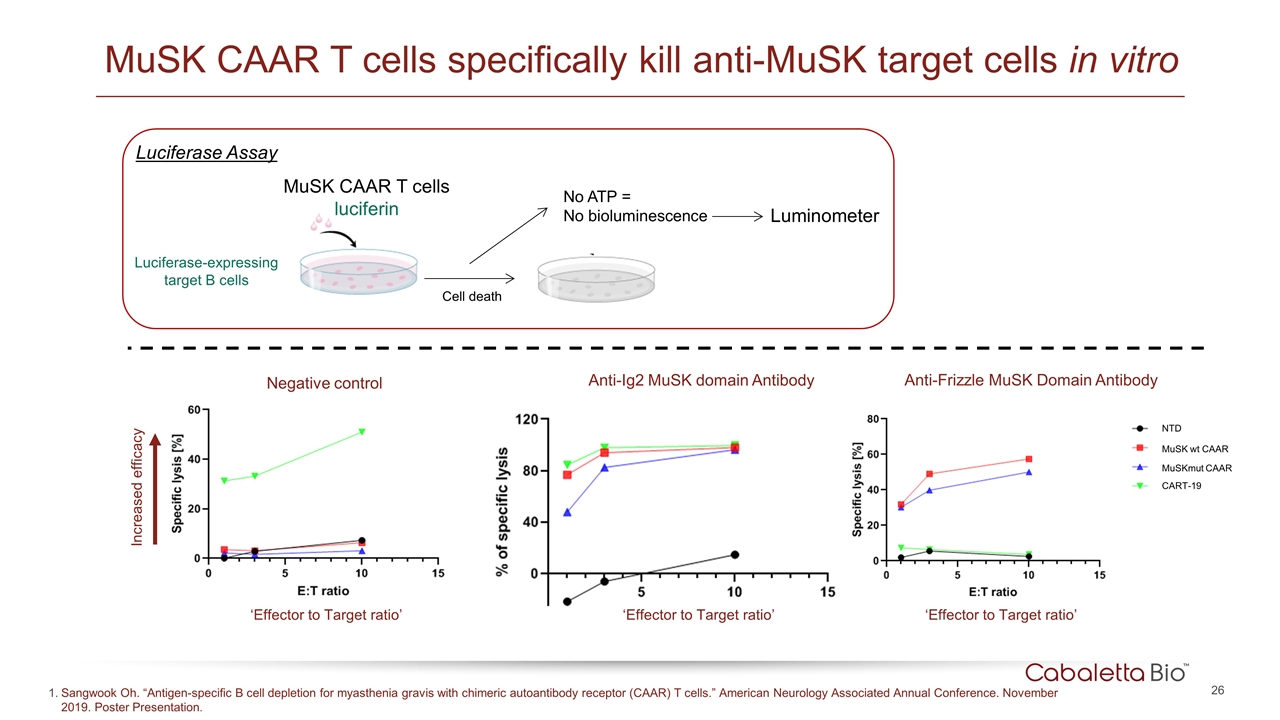
MuSK CAAR T cells specifically kill anti-MuSK target cells in vitro Luciferase-expressing target B cells MuSK CAAR T cells luciferin Cell death No ATP = No bioluminescence Luminometer Luciferase Assay Negative control Anti-Ig2 MuSK domain Antibody Anti-Frizzle MuSK Domain Antibody Increased efficacy ‘Effector to Target ratio’ NTD MuSK wt CAAR MuSKmut CAAR CART-19 ‘Effector to Target ratio’ ‘Effector to Target ratio’ Sangwook Oh. “Antigen-specific B cell depletion for myasthenia gravis with chimeric autoantibody receptor (CAAR) T cells.” American Neurology Associated Annual Conference. November 2019. Poster Presentation.
FVIII-CAART for Hemophilia A inhibitor patients
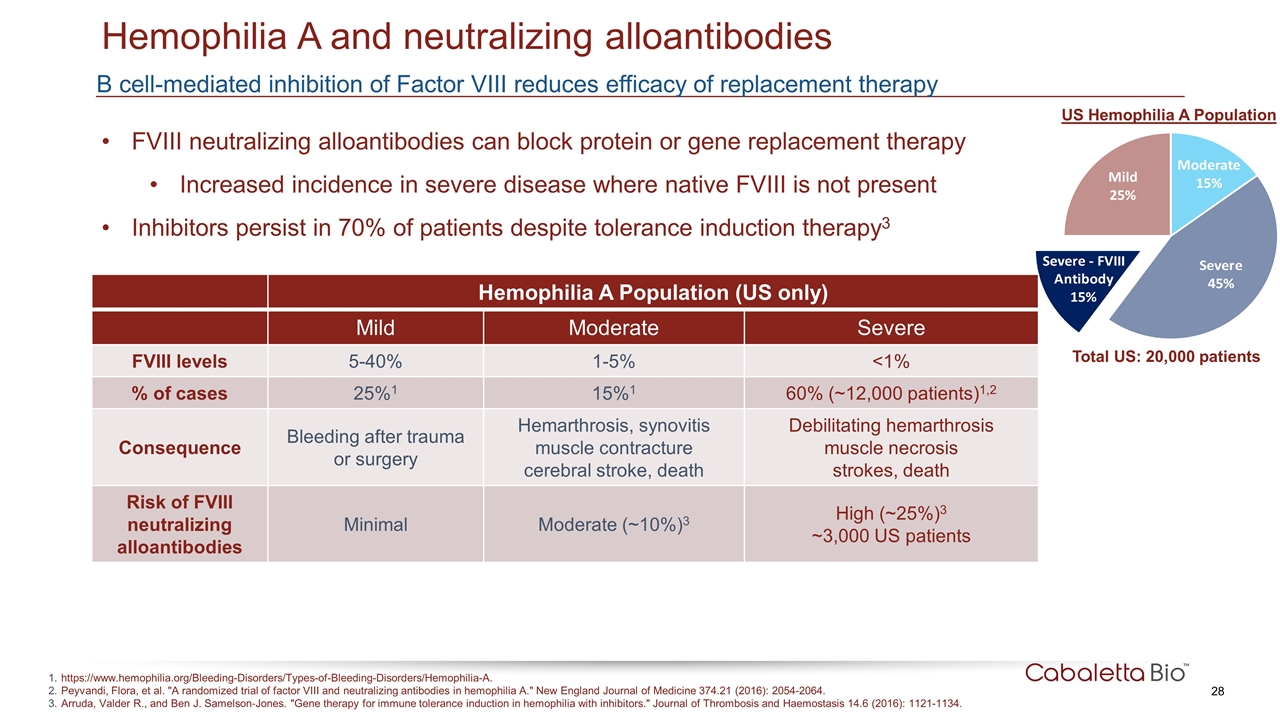
B cell-mediated inhibition of Factor VIII reduces efficacy of replacement therapy Hemophilia A and neutralizing alloantibodies https://www.hemophilia.org/Bleeding-Disorders/Types-of-Bleeding-Disorders/Hemophilia-A. Peyvandi, Flora, et al. "A randomized trial of factor VIII and neutralizing antibodies in hemophilia A." New England Journal of Medicine 374.21 (2016): 2054-2064. Arruda, Valder R., and Ben J. Samelson‐Jones. "Gene therapy for immune tolerance induction in hemophilia with inhibitors." Journal of Thrombosis and Haemostasis 14.6 (2016): 1121-1134. FVIII neutralizing alloantibodies can block protein or gene replacement therapy Increased incidence in severe disease where native FVIII is not present Inhibitors persist in 70% of patients despite tolerance induction therapy3 Hemophilia A Population (US only) Mild Moderate Severe FVIII levels 5-40% 1-5% <1% % of cases 25%1 15%1 60% (~12,000 patients)1,2 Consequence Bleeding after trauma or surgery Hemarthrosis, synovitis muscle contracture cerebral stroke, death Debilitating hemarthrosis muscle necrosis strokes, death Risk of FVIII neutralizing alloantibodies Minimal Moderate (~10%)3 High (~25%)3 ~3,000 US patients US Hemophilia A Population Total US: 20,000 patients
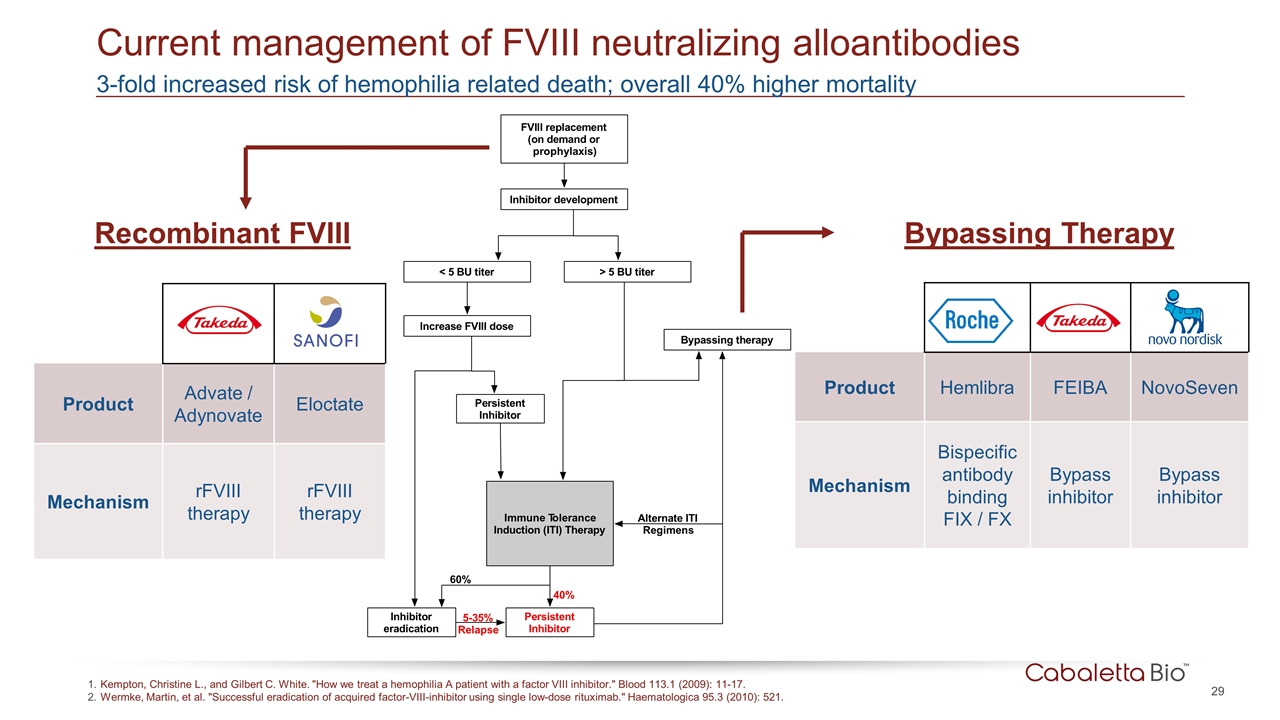
Product Hemlibra FEIBA NovoSeven Mechanism Bispecific antibody binding FIX / FX Bypass inhibitor Bypass inhibitor Product Advate / Adynovate Eloctate Mechanism rFVIII therapy rFVIII therapy 3-fold increased risk of hemophilia related death; overall 40% higher mortality Current management of FVIII neutralizing alloantibodies Kempton, Christine L., and Gilbert C. White. "How we treat a hemophilia A patient with a factor VIII inhibitor." Blood 113.1 (2009): 11-17. Wermke, Martin, et al. "Successful eradication of acquired factor-VIII-inhibitor using single low-dose rituximab." Haematologica 95.3 (2010): 521. Recombinant FVIII Bypassing Therapy F VI I I r e p l a c e m e n t (o n d e m a n d o r prophylaxis) I n h i b i to r d e v e l o p m e n t < 5 B U ti te r > 5 B U ti te r I n c r e a s e F VI I I d o s e B y p a s s i n g th e r a p y Immune T olerance I n d u c ti o n (I T I ) T h e r a p y Pe r s i s te n t I n h i b i to r I n h i b i to r e r a d i c a ti o n Pe r s i s te n t I n h i b i to r A l te r n a te I T I Regimens 60% 40% 5 -3 5 % Relapse
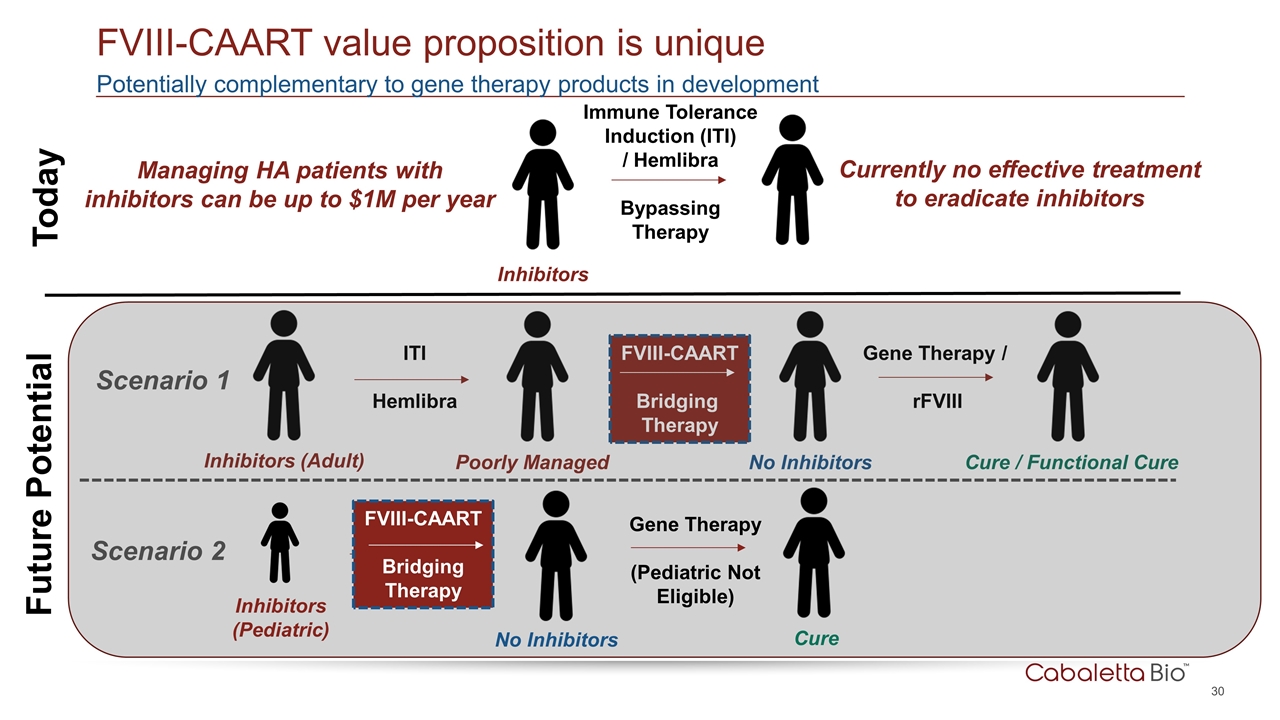
FVIII-CAART Bridging Therapy Potentially complementary to gene therapy products in development FVIII-CAART value proposition is unique Today Future Potential Inhibitors Immune Tolerance Induction (ITI) / Hemlibra Bypassing Therapy ITI Hemlibra Gene Therapy / rFVIII Poorly Managed No Inhibitors Cure / Functional Cure Currently no effective treatment to eradicate inhibitors Managing HA patients with inhibitors can be up to $1M per year Inhibitors (Adult) Scenario 1 Scenario 2 FVIII-CAART Bridging Therapy Gene Therapy (Pediatric Not Eligible) Inhibitors (Pediatric) No Inhibitors Cure
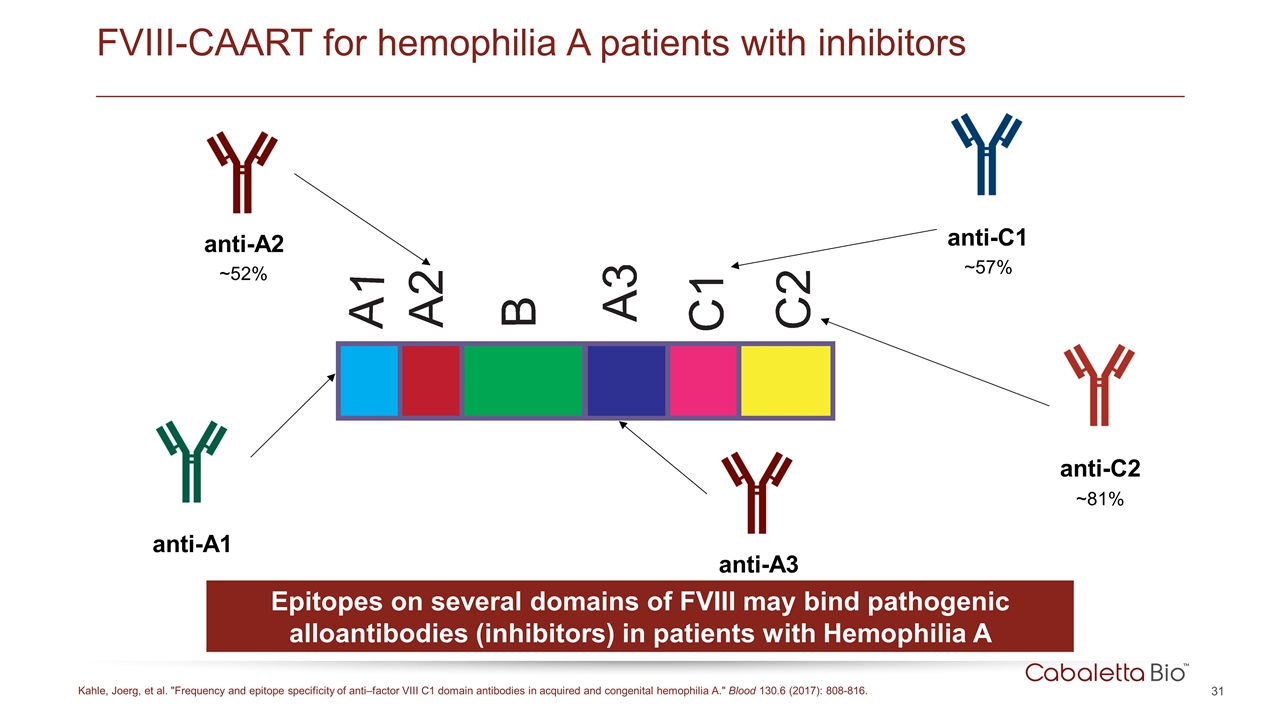
anti-A2 ~52% FVIII-CAART for hemophilia A patients with inhibitors Kahle, Joerg, et al. "Frequency and epitope specificity of anti–factor VIII C1 domain antibodies in acquired and congenital hemophilia A." Blood 130.6 (2017): 808-816. anti-A1 anti-C1 ~57% anti-C2 ~81% Epitopes on several domains of FVIII may bind pathogenic alloantibodies (inhibitors) in patients with Hemophilia A anti-A3
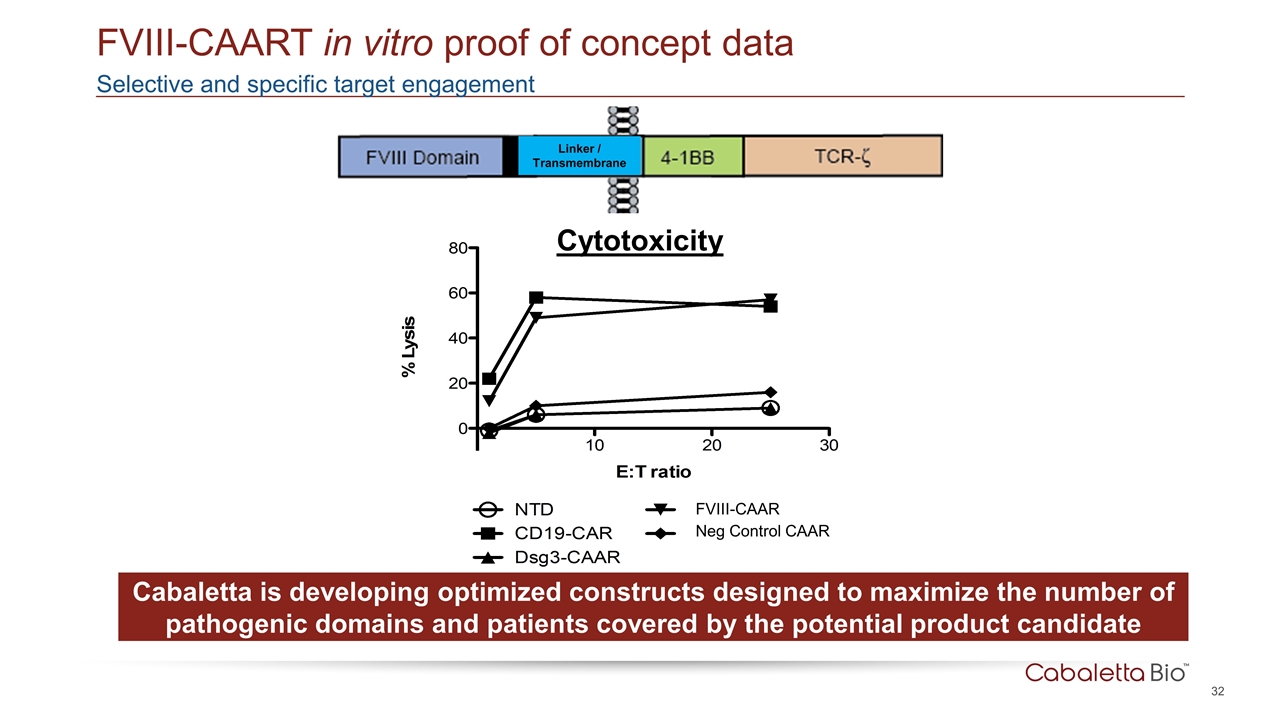
Selective and specific target engagement FVIII-CAART in vitro proof of concept data Cytotoxicity Cabaletta is developing optimized constructs designed to maximize the number of pathogenic domains and patients covered by the potential product candidate FVIII-CAAR Linker / Transmembrane Neg Control CAAR
DSG3/1-CAART for mucocutaneous PV patients
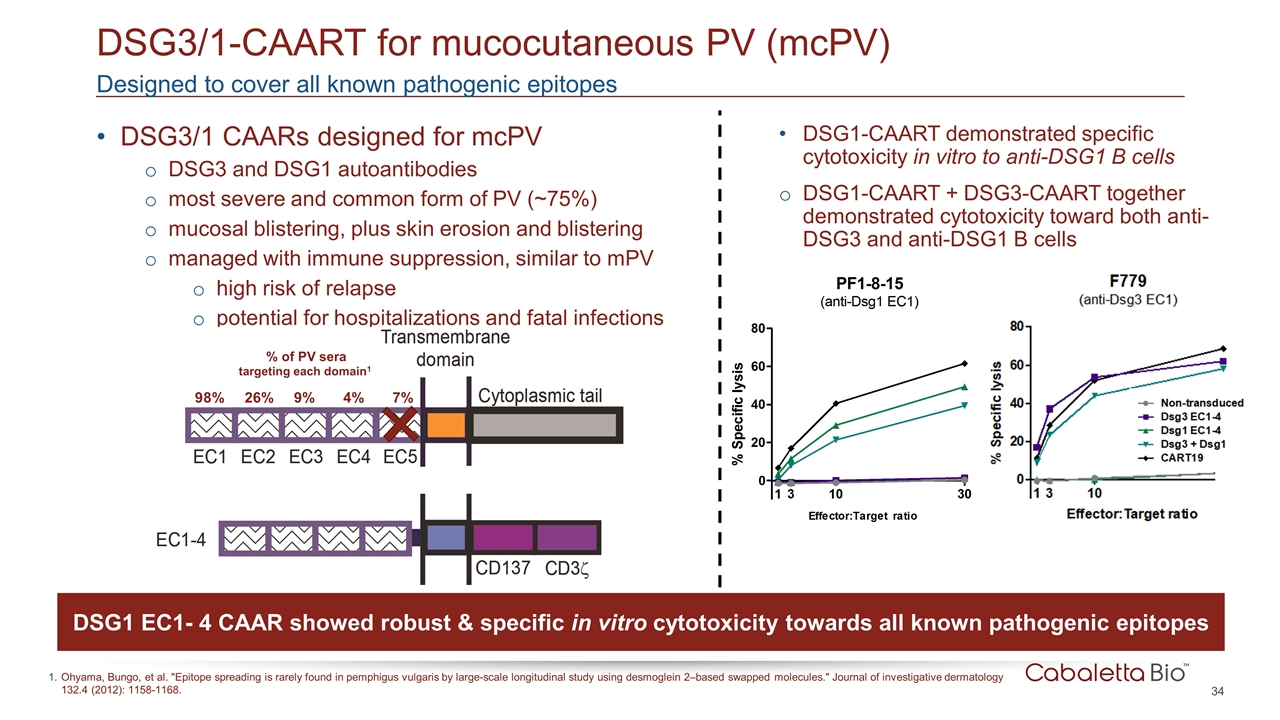
DSG3/1 CAARs designed for mcPV DSG3 and DSG1 autoantibodies most severe and common form of PV (~75%) mucosal blistering, plus skin erosion and blistering managed with immune suppression, similar to mPV high risk of relapse potential for hospitalizations and fatal infections Designed to cover all known pathogenic epitopes DSG3/1-CAART for mucocutaneous PV (mcPV) Ohyama, Bungo, et al. "Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudinal study using desmoglein 2–based swapped molecules." Journal of investigative dermatology 132.4 (2012): 1158-1168. DSG1-CAART demonstrated specific cytotoxicity in vitro to anti-DSG1 B cells DSG1-CAART + DSG3-CAART together demonstrated cytotoxicity toward both anti-DSG3 and anti-DSG1 B cells 9 8 % 26% 9% 4% 7% % of PV sera targeting each domain1 DSG1 EC1- 4 CAAR showed robust & specific in vitro cytotoxicity towards all known pathogenic epitopes
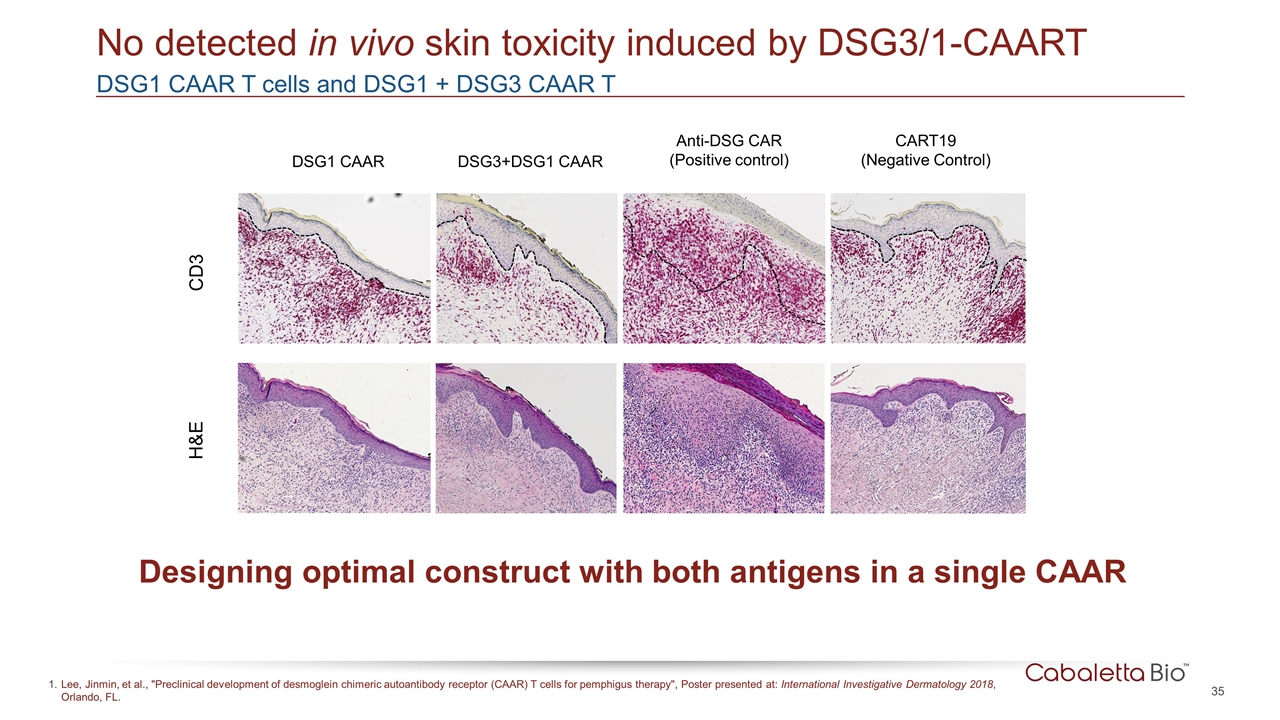
DSG1 CAAR T cells and DSG1 + DSG3 CAAR T No detected in vivo skin toxicity induced by DSG3/1-CAART DSG1 CAAR DSG3+DSG1 CAAR Anti-DSG CAR (Positive control) CART19 (Negative Control) CD3 H&E Designing optimal construct with both antigens in a single CAAR Lee, Jinmin, et al., "Preclinical development of desmoglein chimeric autoantibody receptor (CAAR) T cells for pemphigus therapy", Poster presented at: International Investigative Dermatology 2018, Orlando, FL.

Manufacturing
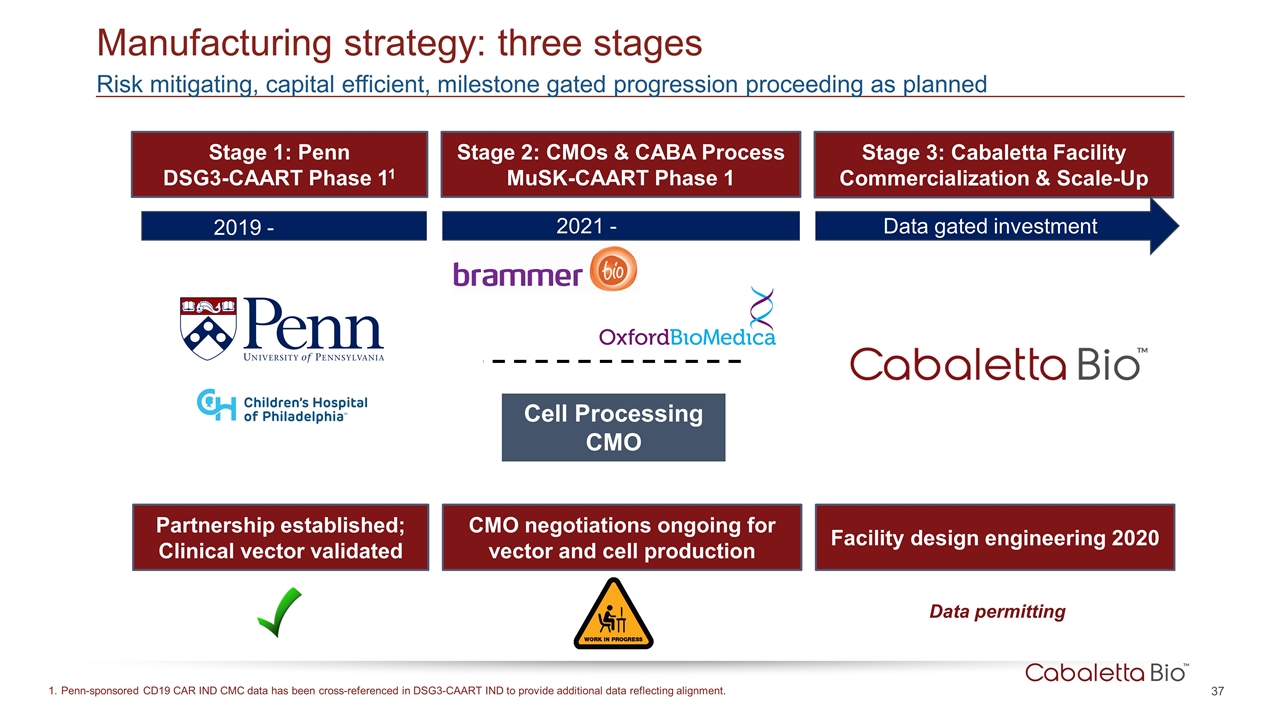
Manufacturing strategy: three stages Risk mitigating, capital efficient, milestone gated progression proceeding as planned Stage 1: Penn DSG3-CAART Phase 11 Stage 2: CMOs & CABA Process MuSK-CAART Phase 1 Stage 3: Cabaletta Facility Commercialization & Scale-Up Data permitting Partnership established; Clinical vector validated CMO negotiations ongoing for vector and cell production Facility design engineering 2020 Penn-sponsored CD19 CAR IND CMC data has been cross-referenced in DSG3-CAART IND to provide additional data reflecting alignment. Cell Processing CMO 2019 - 2021 - Data gated investment
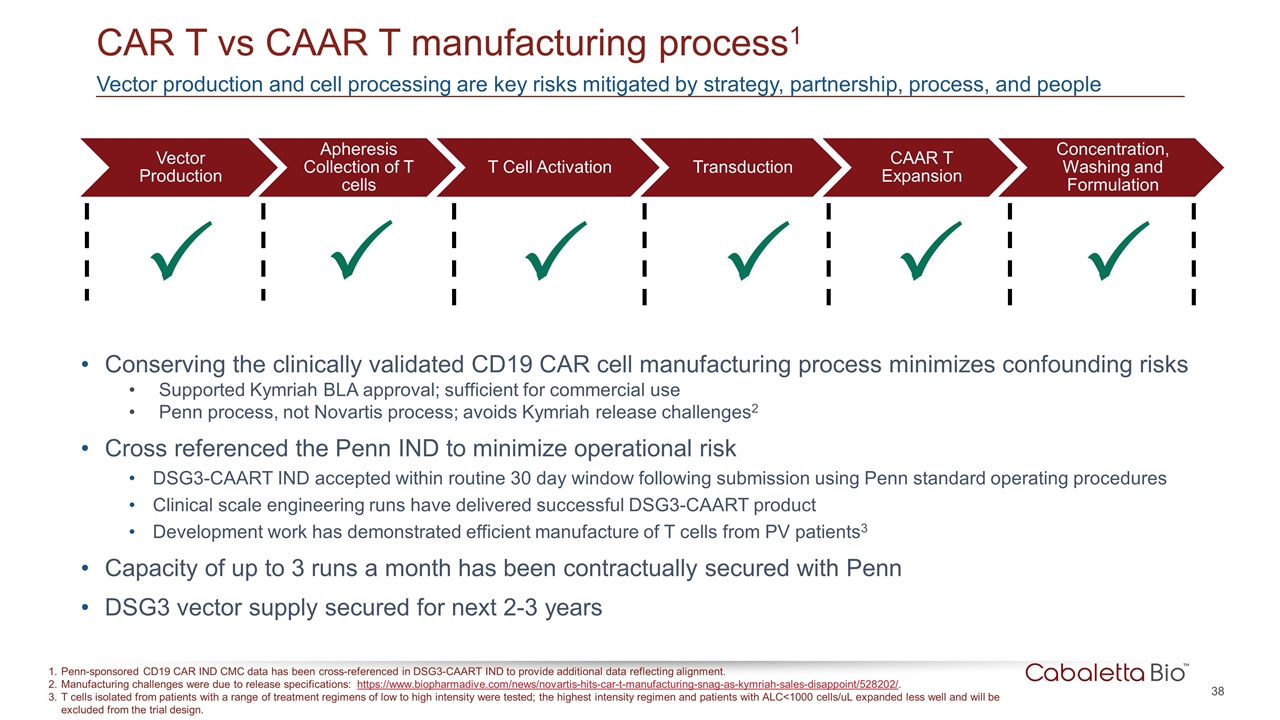
CAR T vs CAAR T manufacturing process1 Penn-sponsored CD19 CAR IND CMC data has been cross-referenced in DSG3-CAART IND to provide additional data reflecting alignment. Manufacturing challenges were due to release specifications: https://www.biopharmadive.com/news/novartis-hits-car-t-manufacturing-snag-as-kymriah-sales-disappoint/528202/. T cells isolated from patients with a range of treatment regimens of low to high intensity were tested; the highest intensity regimen and patients with ALC<1000 cells/uL expanded less well and will be excluded from the trial design. P P P P Vector production and cell processing are key risks mitigated by strategy, partnership, process, and people P P Conserving the clinically validated CD19 CAR cell manufacturing process minimizes confounding risks Supported Kymriah BLA approval; sufficient for commercial use Penn process, not Novartis process; avoids Kymriah release challenges2 Cross referenced the Penn IND to minimize operational risk DSG3-CAART IND accepted within routine 30 day window following submission using Penn standard operating procedures Clinical scale engineering runs have delivered successful DSG3-CAART product Development work has demonstrated efficient manufacture of T cells from PV patients3 Capacity of up to 3 runs a month has been contractually secured with Penn DSG3 vector supply secured for next 2-3 years Vector Production T Cell Activation Transduction CAAR T Expansion Concentration, Washing and Formulation Apheresis Collection of T cells
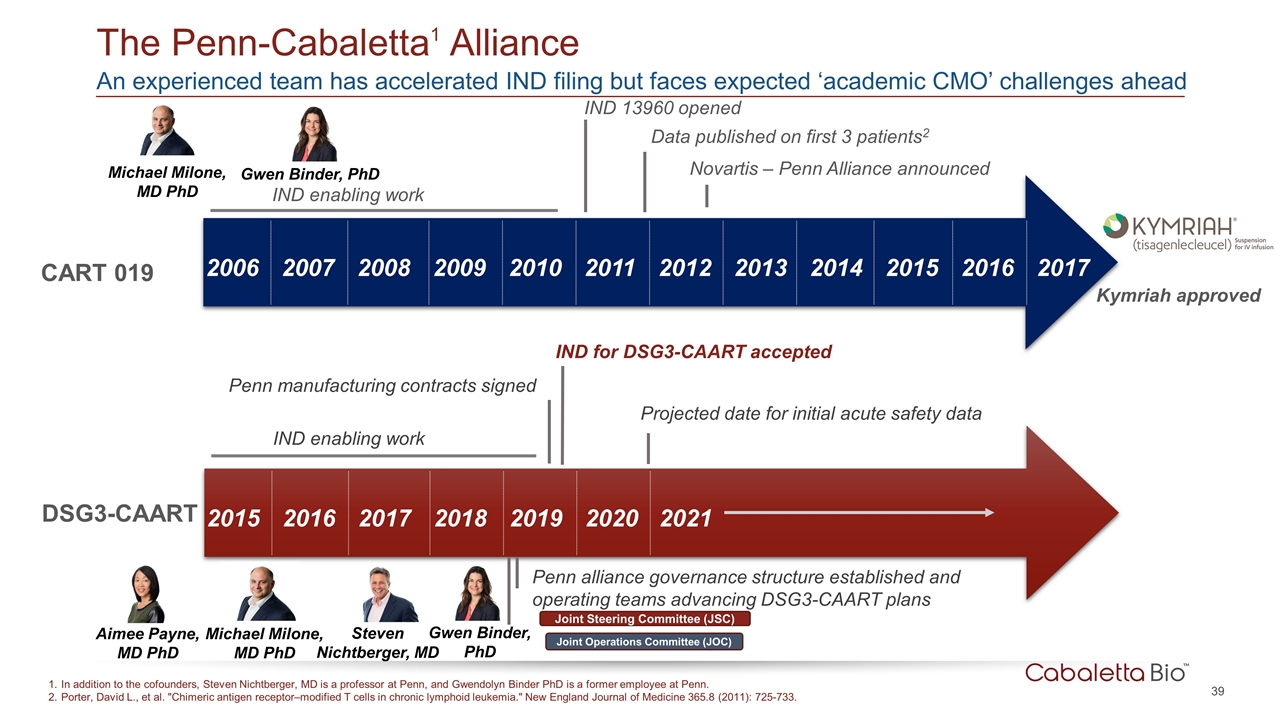
An experienced team has accelerated IND filing but faces expected ‘academic CMO’ challenges ahead The Penn-Cabaletta1 Alliance In addition to the cofounders, Steven Nichtberger, MD is a professor at Penn, and Gwendolyn Binder PhD is a former employee at Penn. Porter, David L., et al. "Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia." New England Journal of Medicine 365.8 (2011): 725-733. 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 CART 019 IND enabling work Data published on first 3 patients2 Novartis – Penn Alliance announced Kymriah approved IND 13960 opened Michael Milone, MD PhD Gwen Binder, PhD Aimee Payne, MD PhD Steven Nichtberger, MD Gwen Binder, PhD 2021 2020 2019 2018 2017 2016 2015 DSG3-CAART IND enabling work IND for DSG3-CAART accepted Projected date for initial acute safety data Penn alliance governance structure established and operating teams advancing DSG3-CAART plans Penn manufacturing contracts signed Michael Milone, MD PhD Joint Steering Committee (JSC) Joint Operations Committee (JOC)

Financial Update
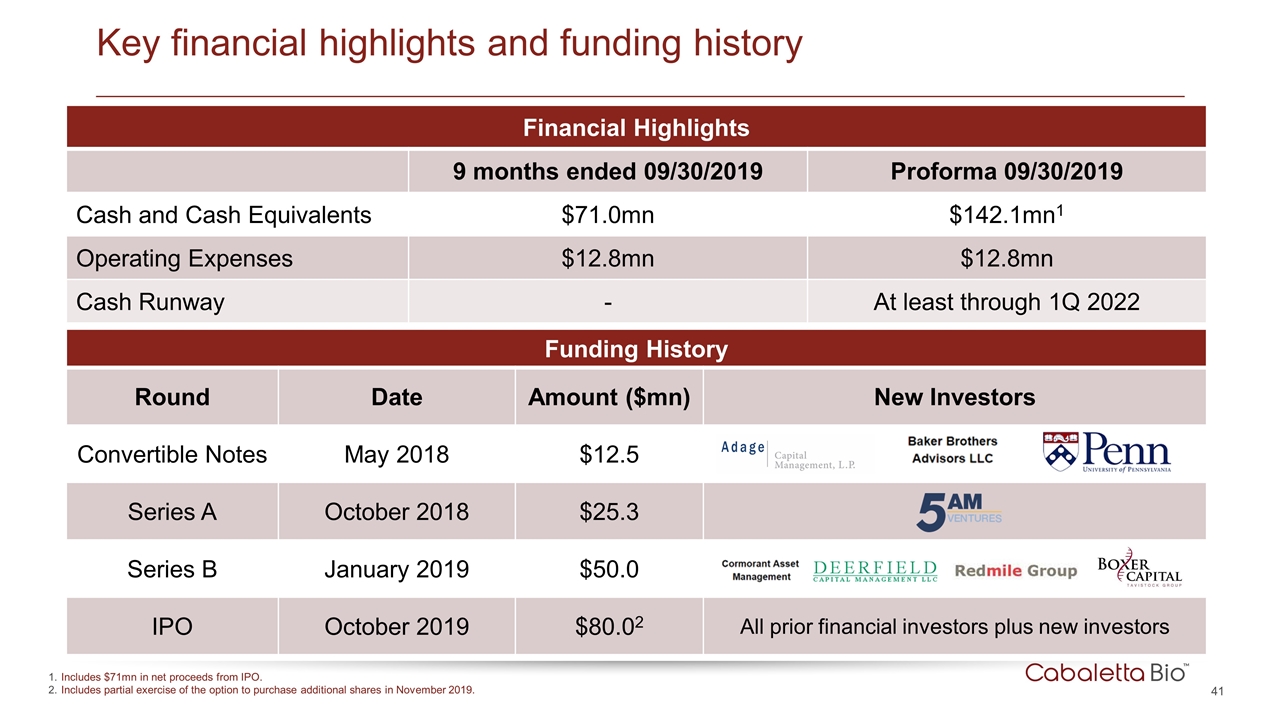
Key financial highlights and funding history Financial Highlights 9 months ended 09/30/2019 Proforma 09/30/2019 Cash and Cash Equivalents $71.0mn $142.1mn1 Operating Expenses $12.8mn $12.8mn Cash Runway - At least through 1Q 2022 Funding History Round Date Amount ($mn) New Investors Convertible Notes May 2018 $12.5 Series A October 2018 $25.3 Series B January 2019 $50.0 IPO October 2019 $80.02 All prior financial investors plus new investors Includes $71mn in net proceeds from IPO. Includes partial exercise of the option to purchase additional shares in November 2019.
2019 Recruited uniquely qualified leadership team with longstanding connections Established broad alliance with Penn to accelerate DSG3-CAART clinical development Secured first issued US product patent and expanded portfolio Expanded Penn license to include filed patents for one additional target Manufactured and qualified vector for clinical trial, completed manufacturing engineering runs IND accepted for DSG3-CAART, IRB process being initiated In vitro MuSK-CAART data presented at scientific meeting 2020 DesCAARTes clinical trial Initiate trial and generate acute tolerability (8 day) data from initial patient cohort MuSK-CAART Present in vivo target engagement data and initiate IND-enabling studies Initiate validation of manufacturing with CMO for clinical program Incorporate advanced technologies as warranted Recent and anticipated catalysts
Selective B cell ablation for autoimmune disease February 2020